Abstract
Glycogen synthase kinase 3 (GSK-3) is implicated in multiple biological processes including metabolism, gene expression, cell fate determination, proliferation, and survival. GSK-3 activity is inhibited through phosphorylation of serine 21 in GSK-3α and serine 9 in GSK-3β. These serine residues of GSK-3 have been previously identified as targets of protein kinase B (PKB/Akt), a serine/threonine kinase located downstream of phosphatidylinositol 3-kinase. Here, we show that serine 21 in GSK-3α and serine 9 in GSK-3β are also physiological substrates of cAMP-dependent protein kinase A. Protein kinase A physically associates with, phosphorylates, and inactivates both isoforms of GSK-3. The results indicate that depending on the stimulatory context, the activity of GSK-3 can be modulated either by growth factors that work through the phosphatidylinositol 3-kinase–protein kinase B cascade or by hormonal stimulation of G protein-coupled receptors that link to changes in intracellular cAMP levels.
Glycogen synthase kinase 3 (GSK-3), a ubiquitously expressed and evolutionarily conserved protein serine/threonine kinase, was originally identified as an enzyme that regulates glycogen synthesis in response to insulin (1). More recent studies implicate GSK-3 in multiple biological processes. GSK-3 phosphorylates a broad range of substrates, including several transcription factors such as c-Myc, c-Jun, and c-Myb (2) and the translation factor eIF2B (3). GSK-3 has also been implicated in the regulation of cell fate in Dictyostelium (4) and is a component of the Wnt signaling pathway required for Drosophila and Xenopus development (5–8). In mammalian cells, on stimulation with insulin or other growth factors, GSK-3 is rapidly phosphorylated at serine 21 in GSK-3α or serine 9 in GSK-3β, resulting in inhibition of GSK-3 kinase activity (9–12). Protein kinase B (PKB/Akt), a serine/threonine kinase located downstream of phosphatidylinositol 3-kinase (PI3K), has been demonstrated to phosphorylate both of these sites in vitro and in vivo, suggesting that growth factors down-regulate GSK-3 activity through the PI3K–PKB signaling cascade (10, 12). Consistent with its position downstream of the PI3K–PKB pathway, GSK-3 activity suppresses cell proliferation and survival (1, 13, 14).
The present study identifies a mode of GSK-3 phosphorylation and inactivation independent of a functional PI3K–PKB pathway. We demonstrate that serine 21 in GSK-3α and serine 9 in GSK-3β are also physiological substrates of cAMP-dependent protein kinase A (PKA). PKA physically associates with, phosphorylates, and inactivates both isoforms of GSK-3. Thus PKA functions as a GSK-3 kinase that, in parallel with PKB, controls the activity of the multifunctional enzyme GSK-3.
Materials and Methods
Reagents.
cAMP analogs [8-bromo-cAMP (8-Br-cAMP) and 8-bromo-cGMP (8-Br-GMP)], forskolin, isoproterenol, 3-isobutyl-1-methylxanthine, and wortmannin were purchased from Sigma. H89 and LY294002 were obtained from Calbiochem. Insulin-like growth factor (IGF)-1 and the PKA peptide inhibitor PKI were from Upstate Biotechnology (Lake Placid, NY). Insulin was from GIBCO/BRL.
Cell Lines.
HEK293 and NIH 3T3 were obtained from American Type Culture Collection. Rat1 cells were provided by Wouter H. Moolenaar (Netherlands Cancer Institute). These cell lines were grown in DMEM supplemented with 10% FBS (Sigma). The cells were frozen at early passages and used for less than 10 weeks in continuous culture.
Plasmids.
GSK-3 expression vectors (pMT2GSK-3α and pcDNA3-HA-GSK-3β) were described previously (11). pcDNA3-PKAc containing the human PKAc cDNA was a gift of H. Wen (University of Texas M. D. Anderson Cancer Center).
Transfection.
HEK293, NIH 3T3, and Rat1 cells were plated in 60-mm dishes. One day later, at around 60% confluence, the cells were transfected (5 μg DNA/dish) by using Fugene 6 according to the protocol of the supplier (Roche Molecular Biochemicals). In cotransfection experiments, the molar ratio of the GSK-3 and PKAc plasmids was adjusted to 1:5 to ensure the PKAc was coexpressed with GSK-3α or -β.
Preparation of Cell Lysates and Western Blotting.
Cells were lysed on ice for 30 min in a lysis buffer containing 1% Triton X-100, 50 mM Hepes, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 100 mM NaF, 10 mM sodium pyrophosphate, 25 mM β-glycerol phosphate, 1 mM DTT, 1 mM sodium vanadate, 1 mM benzamidine, 0.1 μM okadaic acid, 10 μg/ml aprotinin, and 10 μg/ml leupeptin and protease inhibitor mixture (Roche Molecular Biochemicals). Equal amounts of total cellular protein were separated by SDS/PAGE, transferred to immobilon [poly(vinylidene difluoride)], and immunoblotted with antibodies following the protocols provided by manufacturers. Anti-phospho-GSK-3α is a sheep polyclonal IgG reactive with GSK-3α phosphorylated at serine 21 (Upstate Biotechnology). Antiphospho-GSK-3β is a rabbit polyclonal IgG recognizing GSK-3β phosphorylated at serine 9 (New England Biolabs). Anti-GSK-3 is a phosphorylation-independent monoclonal antibody that recognizes both GSK-3α and -β (Upstate Biotechnology). Immunocomplexes were visualized by an enhanced chemiluminescence detection kit (Amersham Pharmacia) by using horseradish peroxidase-conjugated secondary antibodies (Bio-Rad).
In Vitro Kinase Assays.
For measuring GSK-3 activity, approximately 150 μg (for GSK-3α) and 75 μg (for GSK-3β) of total cellular protein were diluted in freshly made lysis buffer and immunoprecipitated with 5 μg anti-GSK-3α (sheep anti-rat GSK-3α; Upstate Biotechnology) or with 0.75 μg anti-GSK-3β (mouse monoclonal anti-rat GSK-3β; Transduction Laboratories, Lexington, KY). After 2 h of rotation at 4°C, protein G-Sepharose was added for another 1.5 h of incubation. Immunoprecipitates were washed twice with lysis buffer and twice with kinase reaction buffer (10 mM 4-morpholinepropanesulfonic acid, pH 7.4/1 mM EDTA/10 mM MgAc/50 mM β-glycerol phosphate/1 mM sodium vanadate/0.5 mM NaF/0.1 μM okadaic acid/1 mM benzamidine/1 μg/ml aprotinin/1 mM DTT). Kinase activity of immunoprecipitated GSK-3 was assayed in a total of 40 μl of reaction buffer supplemented with 62.5 μM phosphoglycogen synthase peptide-2 (Upstate Biotechnology)/20 mM MgCl2/125 μM cold ATP/10 μCi [γ-32P] ATP. After 20 min of incubation at 30°C, reaction mixtures were centrifuged, and 15 μl of the supernatant was spotted onto Whatman P81 phosphocellulose paper. Filters were washed in three changes of 0.75% phosphoric acid, rinsed in acetone, dried, and counted in a liquid scintillation counter.
PKB was immunoprecipitated with a sheep polyclonal anti-rat PKB (Upstate Biotechnology). After immunoprecipitation, PKB kinase activity was determined with a PKB kinase assay kit by using crosstide–paramyosin fusion protein as substrate (New England Biolabs). Phosphorylation of the crosstide–paramyosin substrate at a serine site corresponding to serine 21/9 of GSK-3 was revealed by immunoblotting with a GSK-3 phospho-specific antibody, provided with the kit, that recognizes both GSK-3α phosphorylated at serine 21 and GSK-3β phosphorylated at serine 9.
Analyses of Physical Association Between PKA and GSK-3.
GSK-3 antibodies used for immunoprecipitation are the same as those used in the GSK-3 kinase assays. The anti-GSK-3β antibody crossreacts with human, rat, and mouse GSK-3β, whereas the anti-GSK-3α antibody recognizes human and rat but not murine GSK-3α. Rabbit polyclonal antibodies against PKAc or the PKA regulatory subunit RII (PKA RII) (Santa Cruz Biotechnology) were used for PKAc or PKA RII immunoprecipitation and Western blotting. Immunoprecipitates were washed three times with lysis buffer, twice with a washing buffer (8 mM 4-morpholinepropanesulfonic acid, pH 7.4/0.2 mM EDTA/10 mM MgAc) and analyzed by Western blotting.
PKA Phosphorylation of GSK-3 in Vitro.
PKA phosphorylation of GSK-3 was assessed by using a PKA assay kit according to the protocol provided by the manufacturer (Upstate Biotechnology), except that immunoprecipitated GSK-3α, immunoprecipitated HA-GSK-3β, purified GSK-3α (0.1 unit/reaction) (Upstate Biotechnology), or recombinant GSK-3β (5 units/reaction) (New England Biolabs), instead of Kemptide, were used as substrates. HA-GSK-3β was immunoprecipitated with anti-HA antibody from HEK293 cells transfected with HA-GSK-3β (approximately 180 μg total protein for each reaction), and GSK-3α was immunoprecipitated from the same amount of protein of untransfected HEK293 cells by using the GSK-3α-specific antibody. In both cases, HEK293 cells were starved in serum-free medium for at least 12 h before lysing to decrease the background phosphorylation level of GSK-3. Immunoprecipitates were washed twice with lysis buffer and twice with washing buffer (8 mM 4-morpholinepropanesulfonic acid, pH 7.4/0.2 mM EDTA/10 mM MgAc). PKA activity toward each of these substrates was assayed in a total volume of 50 μl of reaction buffer (20 mM 4-morpholinepropanesulfonic acid, pH 7.2/25 mM glycerol phosphate/5 mM EGTA/1 mM sodium vanadate/1 mM DTT/15 μM MgCl2/100 μM ATP) without or with PKAc (50 ng) (Upstate Biotechnology) or with PKAc in the presence of the PKA peptide inhibitor PKI (1.2 μM) (Upstate Biotechnology). After 20 min at 30°C, the reaction was stopped by adding an equal volume of 2 × SDS sample buffer. PKA-mediated phosphorylation of GSK-3 was analyzed by immunoblotting with GSK-3α and GSK-3β phospho-specific antibodies.
Results and Discussion
cAMP Induces PKA-Dependent Phosphorylation and Inactivation of GSK-3.
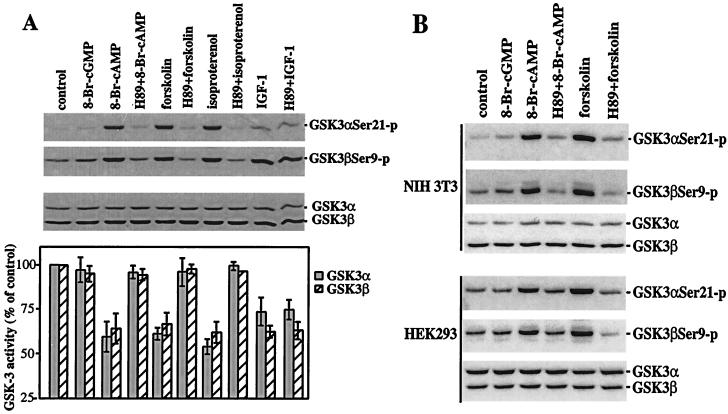
In Rat1, NIH 3T3, and HEK293 cells, increases in the intracellular levels of cAMP stimulate phosphorylation of GSK-3α and -β, as demonstrated by immunoblotting with GSK-3 phosphorylation-specific antibodies. As shown in Fig. 1, the cell-permeable cAMP analogue 8-Br-cAMP induced a marked increase in phosphorylation of GSK-3α and -β at serine 21 and 9, respectively, whereas the structurally related cGMP analogue 8-Br-cGMP had little effect. Forskolin, which activates adenyl cyclase thus raising intracellular cAMP levels (15), triggered a similar elevation in GSK-3 phosphorylation at serine 21 and 9. In Rat1 cells, isoproterenol, which activates the β-adrenergic receptor stimulating adenylate cyclase and increasing endogenous cAMP levels (16, 17), also efficiently stimulated GSK-3 phosphorylation at these serine sites. In NIH 3T3 cells that contain few of the β-adrenergic receptors, stimulation with other G-protein-coupled receptor agonists, such as lysophosphatidic acid. also led to GSK-3 phosphorylation (data not shown).
Figure 1.
PKA-dependent phosphorylation and inactivation of GSK-3 induced by cAMP. Subconfluent Rat1, NIH 3T3, and HEK293 cells were starved in a serum-free medium for 12–24 h and stimulated with 8-Br-cGMP or 8-Br-cAMP (2 mM, 30 min), forskolin (15 μM, 30 min), isoproterenol (10 μM, 30 min), or IGF-1 (75 ng/ml, 10 min). Cells were stimulated with forskolin and isoproterenol in the presence of 0.1 mM of 3-isobutyl-1-methylxanthine to inhibit cellular phosphodiesterase activity. The cell-permeable PKA inhibitor H89 (10 μM) was added to culture where indicated 1 h before stimulation. Cell lysates were prepared and analyzed for GSK-3 phosphorylation at serine 21 and 9 by using GSK-3α and -β phospho-specific antibodies and for GSK-3 kinase assays as described in Materials and Methods. (A) 8-Br-cAMP, forskolin, and isoproterenol induce phosphorylation and inhibition of GSK-3 in a PKA-dependent manner in Rat1 cells. GSK-3 kinase activities are presented as percentage of the activity in unstimulated cells. The results are means ± SD of three independent experiments. (B) 8-Br-cAMP and forskolin stimulate GSK-3 phosphorylation in a PKA-dependent manner in NIH 3T3 and HEK293 cells.
GSK-3 phosphorylation induced by 8-Br-cAMP, forskolin, and isoproterenol was abrogated in the presence of the PKA inhibitor H89 (18), whereas IGF-1-mediated phosphorylation of GSK-3 was insensitive to H89 (Fig. 1), indicating an essential and specific role for PKA in cAMP-initiated phosphorylation of GSK-3 at serine 21 and 9. Consistent with the inhibitory effect of phosphorylation at serine 21 and 9 on GSK-3 activation (9–12), 8-Br-cAMP, forskolin, or isoproterenol dramatically inhibited GSK-3 kinase activity as shown in in vitro kinase assays in Rat1 (Fig. 1A) and HEK293 cells (data not shown).
PKA-Dependent Phosphorylation and Inactivation of GSK-3 Does Not Correlate with Activation of the PI3K–PKB Cascade.
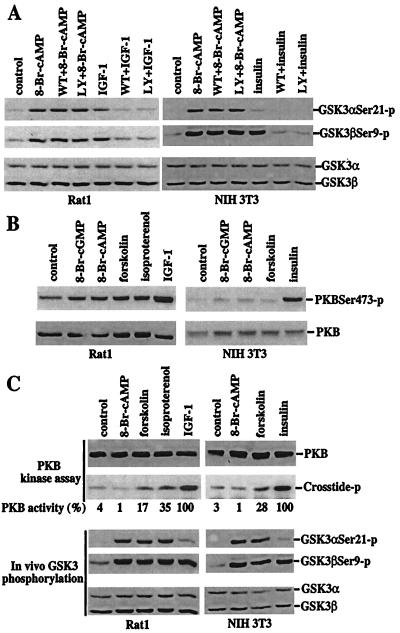
PI3K-dependent activation of PKB is an intermediary in the phosphorylation and inactivation of GSK-3 induced by insulin and other growth factors (10, 12, 14). Insulin and IGF-1 efficiently activate the PI3K pathway in NIH 3T3 and Rat1 cells, respectively. GSK-3 phosphorylation induced by insulin and IGF-1 were eliminated by pretreatment with the PI3K inhibitors wortmannin or LY294002. In contrast, the effects of 8-Br-cAMP or forskolin on GSK-3 phosphorylation in these cell lines were not altered by inhibition of PI3K activity with wortmannin or LY294002 (Fig. 2A), excluding the possibility that PKA induces GSK-3 phosphorylation through activation of PI3K. In some systems, cAMP or PKA has been suggested to induce a PI3K-independent activation of PKB (19). To determine whether PKA-induced PKB activation accounts for GSK-3 phosphorylation after treatment with 8-Br-cAMP, forskolin, or isoproterenol, PKB phosphorylation at serine 473 and threonine 308, which is crucial for activation of PKB (20), was assessed. As expected, insulin and IGF-1 strongly stimulated phosphorylation of PKB at serine 473 in NIH 3T3 or Rat1 cells (Fig. 2B). In contrast, 8-Br-cAMP, forskolin, and isoproterenol did not significantly alter PKB phosphorylation at serine 473 (Fig. 2B). Similar results were obtained with a threonine 308 phosphorylation-specific antibody (data not shown). Furthermore, PKB activity was not increased by treatment of cells with 8-Br-cAMP at concentrations that strongly induced phosphorylation and inactivation of GSK-3 (Fig. 2C). In fact, incubation with 8-Br-cAMP modestly decreased PKB activity because of an as yet undefined mechanism. Forskolin increased PKB activity weakly in Rat1 and NIH 3T3 cells. A slightly stronger stimulation of PKB activity was seen in Rat1 cells treated with isoproterenol. However, activation of PKB by forskolin or isoproterenol was much less than that induced by IGF-1 or insulin (Fig. 2C). As compared with the basal activity present in the unstimulated cells, isoproterenol or forskolin-induced PKB activity was only one-third or less of that stimulated by IGF-1 or insulin. As demonstrated in Fig. 2C, however, 8-Br-cAMP, forskolin, or isoproterenol stimulated phosphorylation of GSK-3α more efficiently than IGF-1 or insulin and increased phosphorylation of GSK-3β to levels comparable to those induced by IGF-1 or insulin. The lack of correlation between PKB activation and GSK phosphorylation in cells after elevation of cAMP levels argues in favor of a PKB-independent route for GSK-3 phosphorylation and inactivation.
Figure 2.
Dissociation of PKA-mediated phosphorylation and inactivation of GSK-3 from a functional PI3K–PKB signaling pathway. (A) PKA-mediated phosphorylation of GSK-3 is independent of PI3K activity. After 12–24 h of incubation in serum-free medium, Rat1 and NIH 3T3 cells were stimulated with 8-Br-cAMP (2 mM, 30 min), IGF-1 (75 ng/ml, 10 min, Rat1), or insulin (0.1 μM, 10 min, NIH 3T3) in the absence or presence of the PI3K inhibitors, wortmannin (125 nM) or LY294002 (12.5 μM). The cells were preincubated with wortmannin or LY294002 for at least 1 h before addition of 8-Br-cAMP, IGF-1, or insulin. Phosphorylation of GSK-3α at serine 21 and GSK-3β at serine 9 was analyzed by immunoblotting with GSK-3 phospho-specific antibodies as in Fig. 1. (B) Elevation of cAMP levels does not increase phosphorylation of PKB at serine 473. Rat1 and NIH 3T3 cells were starved and stimulated with 8-Br-cGMP, 8-Br-cAMP, forskolin, isoproterenol, IGF, or insulin, as detailed in Fig. 1. PKB phosphorylation levels were analyzed by immunoblotting with a PKB phospho-specific antibody that recognizes PKB phosphorylated at serine 473 (New England Biolabs). Reprobing with a PKB antibody reactive with total PKB (New England Biolabs) shows similar loading among samples. (C) 8-Br-cAMP or elevation of endogenous cAMP levels induces no or limited increases in PKB activity. Rat1 and NIH 3T3 cells were stimulated as in B. PKB activity toward the crosstide–paramyosin substrate was determined as detailed in Materials and Methods. The crosstide–paramyosin substrate phosphorylated at a serine site corresponding to serine 21/9 of GSK-3 is indicated with crosstide-p. The intensities of the crosstide-p bands were quantified by densitometry. The values beneath each lane represent relative intensities (%) with bands induced by IGF-1 in Rat1 cells and by insulin in NIH 3T3 cells defined as 100%. For comparison with PKB activity, a fraction of each lysate was analyzed for levels of GSK-3 phosphorylation at serine 21 and 9 by immunoblotting with GSK-3α and -β phospho-specific antibodies (Bottom). Data shown are representative of three independent experiments.
PKA Physically Complexes with GSK-3.
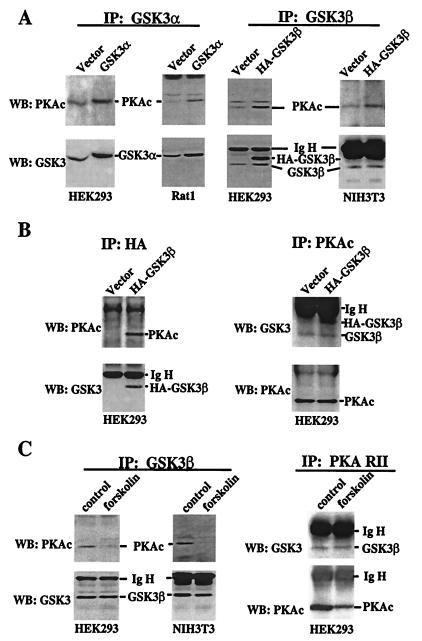
PKA and PKB, a known GSK-3 kinase, are structurally related, exhibiting a high homology especially within their catalytic domains (21–23). The amino acid sequences around serine 21 (SGRARTSSFA) in GSK-3α and serine 9 in GSK-3β (SGRPRTTSFA) comply with the “high-probability” PKA consensus phosphorylation site RXXS/T (24, 25). Further, both GSK-3 and PKA are present in cytosol (1, 25). To explore the possibility that PKA may function as a GSK-3 kinase that directly phosphorylates GSK-3, we examined whether PKA physically interacts with GSK-3 in intact cells. As demonstrated in Fig. 3A, in Rat1 and HEK293, GSK-3α forms a stable complex with the α catalytic subunit of PKA (PKAc) (24), as reflected by the presence of PKAc in GSK-3α immunoprecipitates. The amount of PKAc that coprecipitated with GSK-3α was enhanced by overexpression of GSK-3α in transiently transfected cells, indicating that the ability to coprecipitate PKAc depended on GSK-3α protein levels. The GSK-3α antibody used for immunoprecipitation does not react with murine GSK-3α, precluding assessment of association between GSK-3α and PKAc in NIH 3T3 cells (data not shown). Similar to GSK-3α, GSK-3β immunoprecipitates also contained PKAc (Fig. 3A). Transient transfection with HA-tagged GSK-3β (HA-GSK-3β) increased the amount of PKAc that was coprecipitated with GSK-3β. When the transfected GSK-3β was immunoprecipitated by using an anti-HA epitope tag antibody, PKAc was coprecipitated from HA-GSK-3β-transfected cells but not from control vector-transfected cells (Fig. 3B), indicating a specific association between GSK-3β and PKA. In the reciprocal experiment, both endogenous GSK-3β and transfected HA-GSK-3β were readily detected in PKAc precipitates (Fig. 3B). We were unable to assess whether GSK-3α was present in PKAc precipitates, because GSK-3α (≈52 Kd) (1) colocalizes with the Ig heavy chain. To determine whether the association between GSK-3 and PKAc is regulated by cAMP levels, GSK-3β was immunoprecipitated from HEK293 and NIH 3T3 cells treated with or without forskolin. The level of PKAc in GSK-3β immunoprecipitates was markedly reduced in forskolin-treated cells compared with that in unstimulated cells (Fig. 3C), suggesting that dissociation of the GSK-3β-PKAc complex occurs after PKA activation or GSK-3 phosphorylation. Because GSK-3 also coimmunoprecipitated with the PKA regulatory subunit RII and the association was not affected by forskolin (Fig. 3C), it seems that the association of PKAc with GSK-3 is indirect, mediated by PKA regulatory subunits or A-kinase-anchoring proteins.
Figure 3.
Physical association between PKA and GSK-3. (A) HEK293, Rat1, and NIH 3T3 were transfected with an empty vector or GSK-3 vectors that express rat GSK-3α (pMT2-GSK3α) or HA epitope-tagged human GSK-3β (pcDNA3- HA-GSK3β). Two days after transfection, cells were starved in serum-free medium for 12 h before lysing. GSK-3 was immunoprecipitated (IP) by using isoform-selective GSK-3α or -β antibodies as described in Materials and Methods. The immunocomplex was analyzed by Western blotting (WB) with an anti-PKAc antibody. The membranes were reprobed with an anti-GSK-3 antibody (reactive with both isoforms) to confirm the efficiency and specificity of immunoprecipitation. The locations of PKAc, GSK-3α, GSK-3β, HA-GSK-3β, and the Ig heavy chain (IgH) are indicated. (B) Lysates from vector or HA-GSK-3β-transfected HEK293 cells were immunoprecipitated with anti-HA monoclonal antibody. The immunocomplex was subjected to Western blotting with an anti-PKAc antibody and then with an anti-GSK-3 antibody as in A. In the reciprocal experiment (Right), PKAc was immunoprecipitated from the same lysates. The immunocomplex was analyzed by Western blotting for GSK-3 and then for PKAc. (C) Untransfected HEK293 and NIH 3T3 cells were starved in serum-free medium for 12–24 h and stimulated with forskolin or vehicle (control) as described in Fig. 1. GSK-3β or PKA RII was immunoprecipitated and the immunocomplex subjected to Western blotting with the indicated antibodies.
PKA Phosphorylates GSK-3 in Vivo and in Vitro.
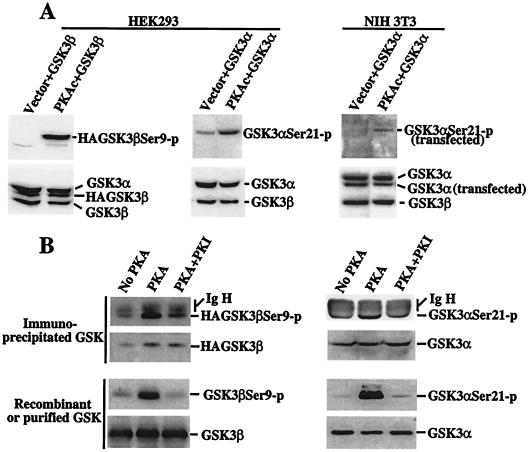
We next assessed the ability of PKA to phosphorylate GSK-3 in vivo and in vitro. Overexpression of PKAc by transient transfection results in constitutive activity in resting cells because of an excess of the catalytic subunit compared with the endogenous regulatory subunits (25). Transfection of PKAc, but not empty vector, enhanced phosphorylation of cotransfected GSK-3α and -β at serine 21 and 9 in HEK293 cells (Fig. 4A). The transfected GSK-3α (human) and cellular GSK-3α in HEK293 migrated at the same location and were not distinguishable. In NIH 3T3 cells, however, the murine GSK-3a was larger and thus separable from the transfected human GSK-3α. PKAc-mediated phosphorylation of coexpressed GSK-3α or GSK-3β was particularly high when compared with the basal phosphorylation levels of endogenous GSK-3, which were low in starved unstimulated cells.
Figure 4.
In vivo and in vitro phosphorylation of GSK-3 by PKA. (A) Transfection of PKAc induces GSK-3 phosphorylation at serine 21 and 9 in intact cells. HEK293 and NIH 3T3 cells were cotransfected with pcDNA3-GSK-3β (labeled as GSK-3β) or pMT2-GSK-3α (GSK-3α) along with an empty vector (Vector) or pcDNA3-PKAc (PKAc). Two days after transfection, cells were starved for 12 h in serum-free medium and lysates prepared. Total cell lysates were analyzed for phosphorylation of transfected and endogenous GSK-3 by immunoblotting with GSK-3α and -β phospho-specific antibodies. Expression of cellular and transfected GSK-3α or -β was determined by immunoblotting with an anti-GSK-3 antibody. The relative locations of cellular and transfected GSK-3α or -β are indicated (Right). (B) PKAc phosphorylates GSK-3 at serine 21 and 9 in vitro. Immunoprecipitated HA-GSK-3β (Top Left), immunoprecipitated GSK-3α (Top Right), recombinant GSK-3β (Bottom Left), and purified GSK-3α (Bottom Right) were used as substrates for PKAc in a kinase reaction. The immunoprecipitates or recombinant/purified GSK-3α or -β were incubated without PKA (No PKA), with PKA (PKA), or with PKA in the presence of the PKA inhibitor PKI (PKA + PKI). Phosphorylation of the GSK-3α or -β substrates by PKA was determined by immunoblotting with GSK-3α and -β phospho-specific antibodies. Levels of GSK-3α or -β substrates were assessed by immunoblotting with an anti-GSK-3 antibody. Bands corresponding to phosphorylated GSK-3α or -β, total GSK-3α or -β, and the Ig heavy chain (IgH) are indicated (Right).
Consistent with this in vivo activity, PKAc enzyme efficiently phosphorylated GSK-3α and -β at serine 21 and 9 in vitro (Fig. 4B), which was blocked by addition of the PKA peptide inhibitor (PKI) (26). Similar results were obtained when immunoprecipitated GSK-3α, immunoprecipitated HA-GSK-3β, purified GSK-3α, or recombinant GSK-3β was used as substrate (Fig. 4B). The crosstide–paramyosin fusion protein, which is a substrate for PKB in in vitro kinase assays, was also highly phosphorylated by PKA at the same serine site that is phosphorylated by PKB (data not shown). In contrast, other serine/threonine kinases, such as MEK-1 and Erk-2, did not phosphorylate the crosstide–paramyosin fusion protein or GSK-3α or -β at serine 21 or 9 in vitro (data not shown).
These in vivo and in vitro observations establish that PKA can directly phosphorylate and inactivate GSK-3. The result is different from the observations in Dictyostelium that cAMP up-regulates GSK-3 activity via cAMP receptor and subsequent activation of the tyrosine kinase ZAK1 (27, 28). The identification of GSK-3 as a downstream target of PKA in mammalian cells suggests an effector role for GSK-3 in cellular responses to cAMP or PKA activation. For example, cAMP has multiple diverse effects on cellular processes such as gene expression, cell cycle control, and cell survival-death decision (25, 29–31). In some systems, elevation of intracellular cAMP levels stimulates cell proliferation and survival through undefined mechanisms (29–31). Our results herein imply that PKA-mediated inhibition of GSK-3 may underlie or contribute to these effects of cAMP considering the prominent established role of GSK-3 activity in the control of cell proliferation and survival (1, 13, 14).
The convergence of the PKA and PKB signaling pathways at GSK-3 suggests that, depending on the environmental context, the activity of GSK-3 can be regulated either by growth factors (e.g., insulin and IGF-1) working through the PI3K-PKB cascade or by hormonal stimulation (e.g., adrenaline) of G protein-coupled receptors that link to changes in intracellular cAMP concentrations. Analogous to GSK-3, other functionally important proteins such as BAD, a proapoptotic member of the Bcl-2 family, and CREB, a nuclear target of PKA, also appear to represent convergence points of multiple signaling pathways (32–37). BAD was originally demonstrated to be phosphorylated at serine 136 by PKB (32, 33). However, BAD can also be inactivated through phosphorylation of serine 112 by Rsk, which is located downstream of mitogen-activated protein kinases (MAPK/Erk) (34–36), or alternatively by mitochondria-anchored PKA (37). Additionally, Rsk can phosphorylate CREB at the same serine 133 site as PKA, leading to the expression of cAMP-responsive genes (34). Our present work underscores the multiple levels of crosstalk among signaling cascades that regulate functionally critical molecules such as GSK-3.
Acknowledgments
This work was supported by National Institutes of Health Grants CA82716 and CA64602 to G.B.M. and by Atairgin Technologies Research Grant LS99–225RG to X.F. We are grateful to Dr. B. Su (M. D. Anderson Cancer Center) for providing anti-HA monoclonal antibody and to Dr. G. Gallick (M. D. Anderson Cancer Center) for his advice on the PKB kinase assay.
Abbreviations
- GSK-3
glycogen synthase kinase 3
- PKA
protein kinase A
- PKB
protein kinase B
- PI3K
phosphatidylinositol 3-kinase
- 8-Br-cAMP
8-bromo-cAMP
- 8-Br-cGMP
8-bromo-cGMP
- IGF-1
insulin-like growth factor 1.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220413597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220413597
References
- 1.Hughes K, Nikolakaki E, Plyte S E, Totty N F, Woodgett J R. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Biochim Biophys Acta. 1992;1992:1–15. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 3.Welsh G L, Welson C, Proud C G. Trends Cell Biol. 1996;6:274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 4.Harwood A J, Plyte S E, Woodgett J R, Strutt H, Kay R R. Cell. 1995;80:139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 5.Siegfried E, Chou T B, Perrimon N. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- 6.He X, Saint-Jeannet J P, Woodgett J R, Varmus H E, Dawid I B. Nature (London) 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 7.Pierce S B, Kimelman D. Development (Cambridge, UK) 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez I, Itoh K, Sokol S Y. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland C, Leighton I A, Cohen P. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross D A E, Alessi D R, Vandenheede J R, McDowell H E, Hundal H S, Cohen P. Biochem J. 1994;303:21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stambolic V, Woodgett J R. Biochem J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–788. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 13.Cui H, Meng Y, Bulleit R F. Brain Res Dev Brain Res. 1998;111:177–188. doi: 10.1016/s0165-3806(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 14.Pap M, Cooper G M. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 15.Seamon K B, Padgett W, Daly J W. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 17.Hordijk P L, Verlaan I, Jalink K, van Corven E J, Moolenaar W H. J Biol Chem. 1994;269:3534–3538. [PubMed] [Google Scholar]
- 18.Findik D, Song Q, Hidaka H, Lavin M. J Cell Biochem. 1995;57:12–21. doi: 10.1002/jcb.240570103. [DOI] [PubMed] [Google Scholar]
- 19.Filippa N, Sable C L, Filloux C, Hemmings B, Obberghen E V. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 21.Coffer P J, Woodgett J R. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:271–274. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 24.Dell'Acqua M L, Scott J D. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S S, Knighton D R, Zheng J, Sowadski J M, Gibbs C S, Zoller M J. Trends Biochem Sci. 1993;18:84–89. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- 26.Glass D B, Lundquist L J, Katz B M, Walsh D A. J Biol Chem. 1989;264:14579–14584. [PubMed] [Google Scholar]
- 27.Kim L, Liu J, Kimmel A R. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 28.Plyte S E, O'Donovan E, Woodgett J R, Harwood A J. Development (Cambridge, UK) 1999;126:325–333. doi: 10.1242/dev.126.2.325. [DOI] [PubMed] [Google Scholar]
- 29.Dumont J E, Jauniaux B W, Roger P P. Trends Biochem Sci. 1989;14:67–71. doi: 10.1016/0968-0004(89)90046-7. [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Franke A, Kaplan M R, Pfrieger F W, Barres B A. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 31.Von Knethen A, Lotero A, Brune B. Oncogene. 1998;16:387–394. doi: 10.1038/sj.onc.1201926. [DOI] [PubMed] [Google Scholar]
- 32.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 33.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 34.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 35.Fang X, Yu S, Eder A, Mao M, Bast R C, Jr, Boyd D, Mills G B. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 36.Scheid M P, Schubert K M, Duronio V. J Biol Chem. 1999;174:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 37.Harada H, Becknell B, Wilm M, Mann M, Huang L J, Taylor S S, Scott J D, Korsmeyer S J. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]