Abstract
BACKGROUND/AIM—Helicobacter pylori infections are associated with hypochlorhydria in patients with pangastritis. It has previously been shown that eradication of H pylori leads to an increase in acid secretion in H pylori associated enlarged fold gastritis, suggesting that H pylori infection affects parietal cell function in the gastric body. The aim of this study was to evaluate the effects of H pylori infection on parietal cell morphology and function in hypochlorhydric patients. PATIENTS/METHODS—The presence of H pylori infection, mucosal length, and inflammatory infiltration were investigated in six patients with enlarged fold gastritis and 12 patients without enlarged folds. Parietal cell morphology was examined by immunohistochemistry using an antibody against the α subunit of H+,K+-ATPase and electron microscopy. In addition, gastric acid secretion and fasting serum gastrin concentration were determined before and after the eradication of H pylori. RESULTS—In the H pylori positive patients with enlarged fold gastritis, fold width, foveolar length, and inflammatory infiltration were increased. In addition, the immunostaining pattern of H+, K+-ATPase was less uniform, and the percentage of altered parietal cells showing dilated canaliculi with vacuole-like structures and few short microvilli was greatly increased compared with that in H pylori positive patients without enlarged folds. After eradication, fold width, foveolar length, and inflammatory infiltrates decreased and nearly all parietal cells were restored to normal morphology. On the other hand, altered parietal cells were negligible in H pylori negative patients. In addition, the basal acid output and tetragastrin stimulated maximal acid output increased significantly from 0.5 (0.5) to 4.1 (1.5) mmol/h and from 2.5 (1.2) to 13.8 (0.7) mmol/h (p<0.01), and fasting serum gastrin concentrations decreased significantly from 213.5 (31.6) to 70.2 (7.5) pg/ml (p<0.01) after eradication in patients with enlarged fold gastritis. CONCLUSION—The morphological changes in parietal cells associated with H pylori infection may be functionally associated with the inhibition of acid secretion seen in patients with enlarged fold gastritis. Keywords: Helicobacter pylori; enlarged fold gastritis; parietal cell morphology; acid secretion; gastrin
Full Text
The Full Text of this article is available as a PDF (217.0 KB).
Figure 1 .
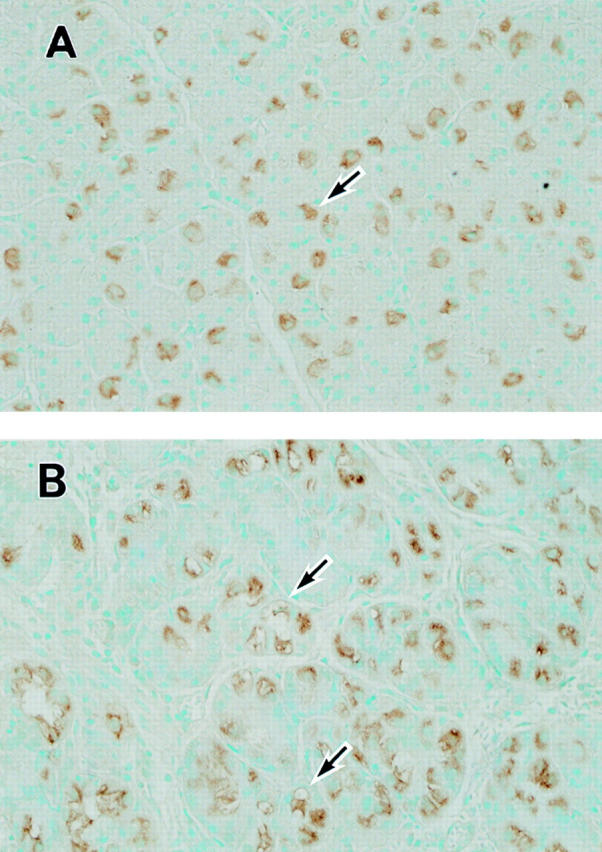
Immunohistochemical detection of H+,K+-ATPase in human gastric mucosa. (A) Gastric mucosal tissue of an H pylori negative patient without enlarged folds. Parietal cell cytoplasm is specifically and uniformly stained (arrow). Original magnification × 210. (B) Gastric mucosal tissue of an H pylori positive patient with enlarged fold gastritis. Some parietal cells are uniformly stained as above. In other parietal cells, stained cytoplasm is less uniform and appears granular or contains several vacuole-like clear areas which are negatively stained for H+,K+-ATPase (arrows). Original magnification × 210.
Figure 2 .
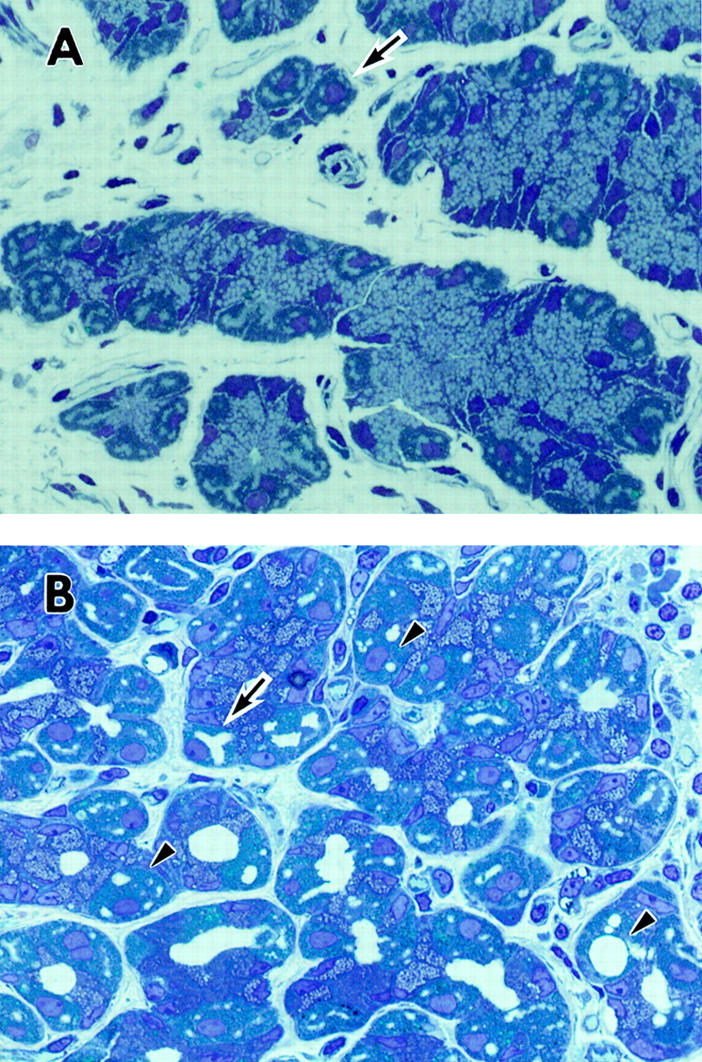
Semithin plastic sections of gastric mucosa stained with toluidine blue. (A) Gastric mucosal tissue from an H pylori negative patient without enlarged folds. Parietal cells appear large and pyramidal or spherical with central nuclei (arrow). Original magnification × 210. (B) Gastric mucosal tissue from an H pylori positive patient with enlarged fold gastritis. Extensive infiltration of inflammatory cells in the lamina propria is visible. Numerous parietal cells show abnormally dilated canaliculi (arrow) and sometimes vacuole-like structures which are different from canaliculi (arrowhead). Original magnification × 210.
Figure 3 .
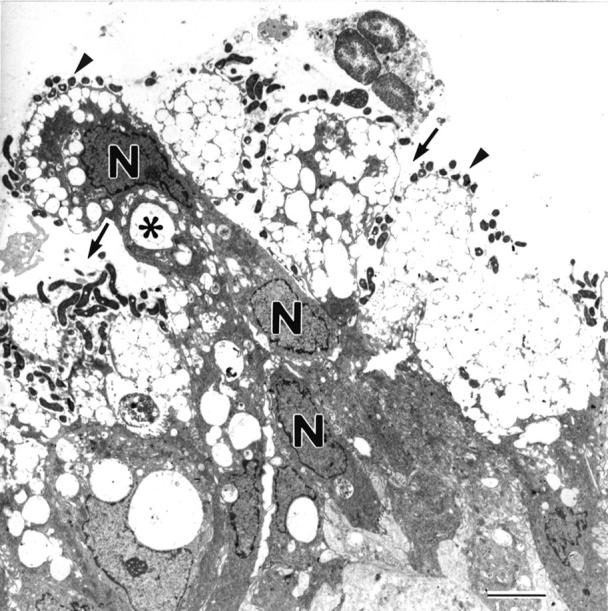
Low magnification electron micrograph of the gastric mucosal lumen in an H pylori positive patient with enlarged fold gastritis. N, nucleus. Numerous bacteria (arrowheads) are observed in the mucous layer and close to gastric epithelial cells. There is considerable superficial vacuolation (*), evidence of loss of microvilli, and depletion of mucin granules. The organisms are also observed in the foveolar and intercellular spaces (arrows). Original magnification × 2000 (bar = 5 µm).
Figure 4 .
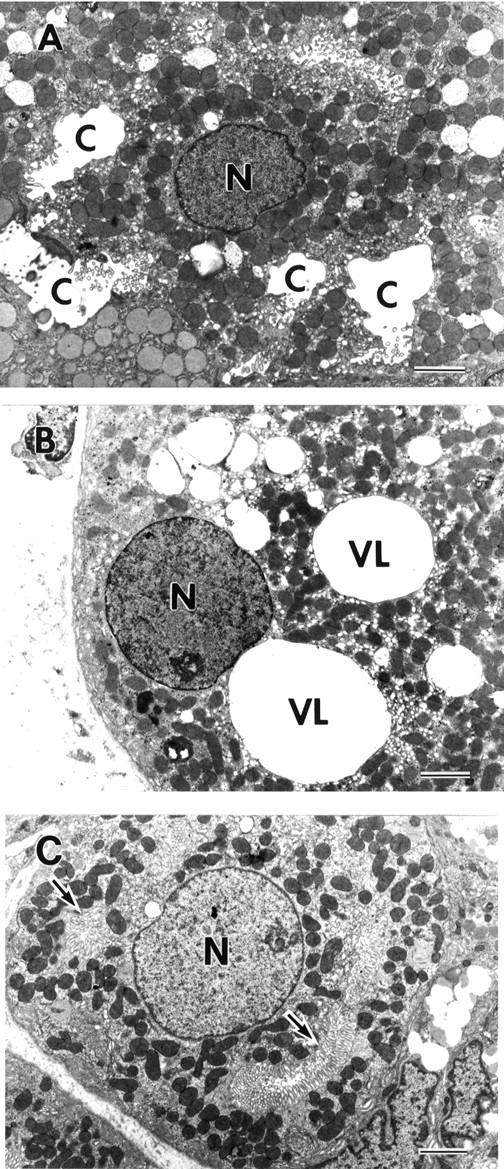
Electron micrographs of parietal cells in an H pylori positive patient with enlarged fold gastritis before (A, B) and after (C) H pylori eradication. N, nucleus; C, intracellular canaliculus. (A) This parietal cell is classified as an altered parietal cell with dilated canaliculi. It has dilated canaliculi containing few short microvilli (bar = 3 µm). (B) This parietal cell is classified as an altered parietal cell with vacuoles. Three large spherical electron lucent structures interpreted to be a vacuole-like structure (VL) are present in the cytoplasm, and microvilli at the luminal surface have completely disappeared (bar = 3 µm). (C) After eradication of H pylori in patients with enlarged fold gastritis, the parietal cell has almost reverted to a normal appearance as seen in H pylori negative patients. Most of the cytoplasm is occupied by tubulovesicles and mitochondria, and canaliculi with developed microvilli can be recognised (arrows) (bar = 3µm). Original magnification: A, × 4000; B, × 4000; C, × 4000.
Figure 5 .
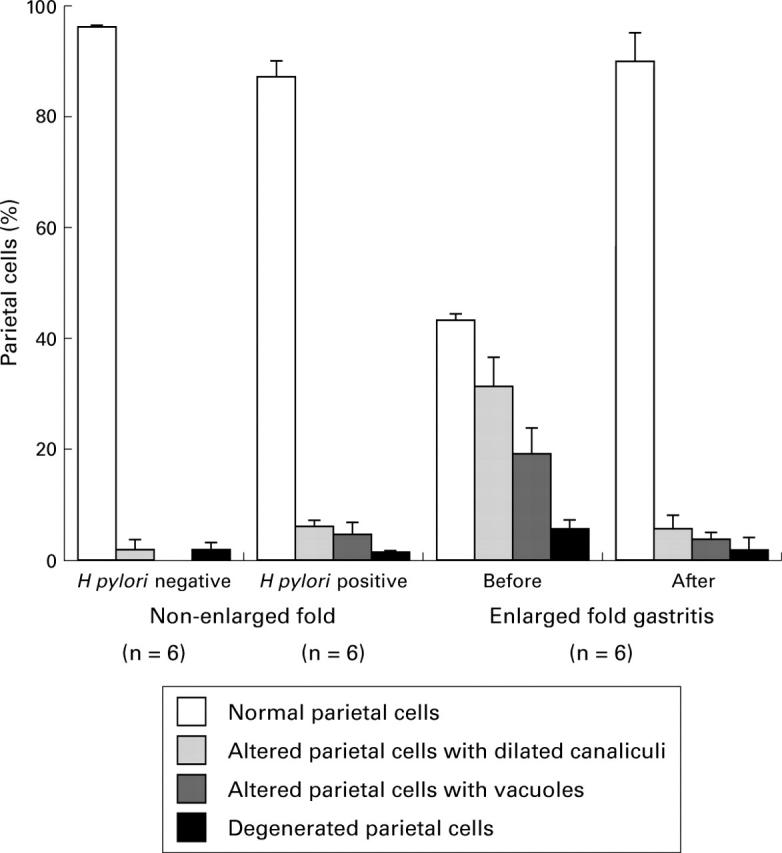
Semiquantitative analysis of parietal cell morphology before and after eradication of H pylori in six patients with enlarged fold gastritis and 12 patients without enlarged folds (six H pylori positive and six H pylori negative). Electron micrographs of gastric mucosa were scored according to parietal cell morphology as being normal, altered with dilated canaliculi, altered with vacuoles, or degenerated. The mean of the percentage of each class is represented by bars. Standard errors are represented by vertical lines.
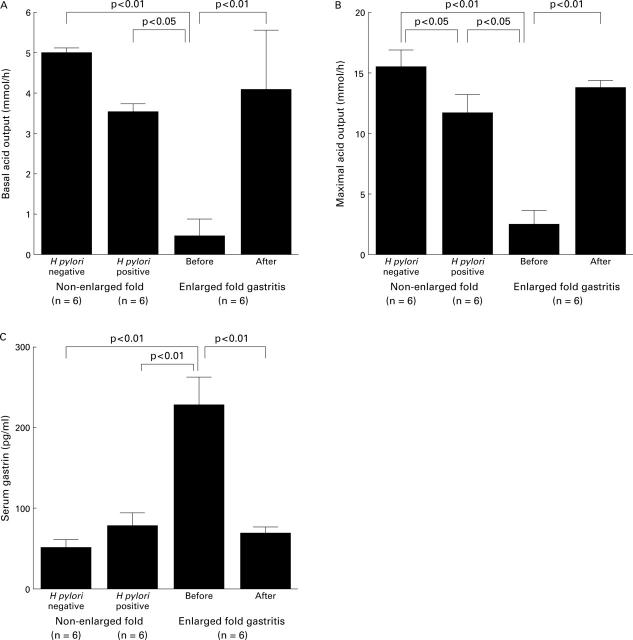
Figure 6 .
Basal acid output (A), 4 µg/kg tetragastrin stimulated maximal acid output (B), and fasting serum gastrin concentrations (C) before and after eradication of H pylori in six patients with enlarged fold gastritis, in six H pylori positive patients without enlarged folds, and six H pylori negative patients.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayerdörffer E., Ritter M. M., Hatz R., Brooks W., Ruckdeschel G., Stolte M. Healing of protein losing hypertrophic gastropathy by eradication of Helicobacter pylori--is Helicobacter pylori a pathogenic factor in Ménétrier's disease? Gut. 1994 May;35(5):701–704. doi: 10.1136/gut.35.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil W., Birkholz C., Wagner S., Sewing K. F. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K(+)-ATPase. Gut. 1994 Sep;35(9):1176–1180. doi: 10.1136/gut.35.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave D. R., Vargas M. Effect of a Campylobacter pylori protein on acid secretion by parietal cells. Lancet. 1989 Jul 22;2(8656):187–189. doi: 10.1016/s0140-6736(89)90372-3. [DOI] [PubMed] [Google Scholar]
- Chen X. G., Correa P., Offerhaus J., Rodriguez E., Janney F., Hoffmann E., Fox J., Hunter F., Diavolitsis S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol. 1986 Nov;86(5):575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996;41:85–117. [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Faller G., Steininger H., Kränzlein J., Maul H., Kerkau T., Hensen J., Hahn E. G., Kirchner T. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997 Nov;41(5):619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology. 1977 Oct;73(4 Pt 2):941–955. [PubMed] [Google Scholar]
- Haot J., Hamichi L., Wallez L., Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988 Sep;29(9):1258–1264. doi: 10.1136/gut.29.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Fujita S. Tritiated thymidine autoradiographic study on cellular migration in the gastric gland of the golden hamster. Cell Tissue Res. 1976 Sep 14;172(2):171–184. doi: 10.1007/BF00226025. [DOI] [PubMed] [Google Scholar]
- Helander H. F., Hirschowitz B. I. Quantitative ultrastructural studies on inhibited and on partly stimulated gastric parietal cells. Gastroenterology. 1974 Sep;67(3):447–452. [PubMed] [Google Scholar]
- Helander H. F. Parietal cell structure during inhibition of acid secretion. Scand J Gastroenterol Suppl. 1984;101:21–26. [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey K. J., Tarnawski A., Krause W. J., Stachura J., Sherman D., Burks M. Effect of pentagastrin on parietal cell ultrastructure in glucagon-pretreated subjects. Dig Dis Sci. 1982 May;27(5):394–400. doi: 10.1007/BF01295646. [DOI] [PubMed] [Google Scholar]
- Karam S. M. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993 Jun;236(2):314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- Komorowski R. A., Caya J. G. Hyperplastic gastropathy. Clinicopathologic correlation. Am J Surg Pathol. 1991 Jun;15(6):577–585. doi: 10.1097/00000478-199106000-00006. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Kawabata S., Miyazaki Y., Imamura I., Fukui H., Matsuzawa Y. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994 Dec;267(6 Pt 1):G966–G971. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- Li H., Helander H. F. Parietal cell kinetics after administration of omeprazole and ranitidine in the rat. Scand J Gastroenterol. 1995 Mar;30(3):205–209. doi: 10.3109/00365529509093264. [DOI] [PubMed] [Google Scholar]
- Logan R. P., Walker M. M., Misiewicz J. J., Gummett P. A., Karim Q. N., Baron J. H. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995 Jan;36(1):12–16. doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R., Francis G. J., Langton S. R., Goodwin C. S., Blincow E. D. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987 Mar;82(3):200–210. [PubMed] [Google Scholar]
- Morrison S., Dahms B. B., Hoffenberg E., Czinn S. J. Enlarged gastric folds in association with Campylobacter pylori gastritis. Radiology. 1989 Jun;171(3):819–821. doi: 10.1148/radiology.171.3.2717759. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Miyagawa J., Higashiyama S., Kondo S., Yabu M., Isozaki K., Kayanoki Y., Kanayama S., Shinomura Y., Taniguchi N. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology. 1995 Oct;109(4):1051–1059. doi: 10.1016/0016-5085(95)90562-6. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Press A. J. Practical significance of gastric rugal folds. Am J Roentgenol Radium Ther Nucl Med. 1975 Sep;125(1):172–183. doi: 10.2214/ajr.125.1.172. [DOI] [PubMed] [Google Scholar]
- Price A. B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Robert A., Olafsson A. S., Lancaster C., Zhang W. R. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci. 1991;48(2):123–134. doi: 10.1016/0024-3205(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Stolte M., Bätz C., Eidt S. Giant fold gastritis--a special form of Helicobacter pylori associated gastritis. Z Gastroenterol. 1993 May;31(5):289–293. [PubMed] [Google Scholar]
- Taguchi Y., Kaito M., Gabazza E. C., Takaji S., Shibata T., Oka S., Ikemura N., Nakao K., Hashimoto Y., Imoto I. Helicobacter pylori inhibits the secretory activity of gastric parietal cells in patients with chronic gastritis. An ultrastructural study. Scand J Gastroenterol. 1997 Jul;32(7):656–663. doi: 10.3109/00365529708996514. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Ivey K. J., Krause W. J., Stachura J., McGuigan J. E., Kolts B. E., Sherman D., Burks M. Effect of secretin on gastric parietal cell ultrastructure in man. Lab Invest. 1982 Jan;46(1):33–38. [PubMed] [Google Scholar]
- Wallace J. L., Cucala M., Mugridge K., Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991 Oct;261(4 Pt 1):G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- Wang X. H., Miyazaki Y., Shinomura Y., Moriyama Y., Nakamoto R. K., Matsuzawa Y., Maeda M., Futai M. Characterization of human autoantibodies reactive to gastric parietal cells. Biochem Biophys Res Commun. 1993 Jan 15;190(1):207–214. doi: 10.1006/bbrc.1993.1032. [DOI] [PubMed] [Google Scholar]
- Winawer S. J., Lipkin M. Cell proliferation kinetics in the gastrointestinal tract of man. IV. Cell renewal in the intestinalized gastric mucosa. J Natl Cancer Inst. 1969 Jan;42(1):9–17. [PubMed] [Google Scholar]
- Wolfsen H. C., Carpenter H. A., Talley N. J. Menetrier's disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993 May;104(5):1310–1319. doi: 10.1016/0016-5085(93)90339-e. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Radioimmunoassay of gastrin. Gastroenterology. 1970 Jan;58(1):1–14. [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Kondo S., Murayama Y., Nishibayashi H., Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996 Dec;39(6):787–794. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Yabu M., Nakanishi T., Miyazaki Y., Murayama Y., Bonilla-Palacios J. J., Matsuzawa Y. Improved fold width and increased acid secretion after eradication of the organism in Helicobacter pylori associated enlarged fold gastritis. Gut. 1994 Nov;35(11):1571–1574. doi: 10.1136/gut.35.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]