Abstract
BACKGROUND—K-ras mutation is one of the first genetic alterations in classical colorectal carcinogenesis. AIMS—To investigate the role of K-ras mutations in carcinogenesis, in long standing ulcerative colitis. METHODS—A total of 161 microdissected and 100 DNA samples from 13 patients were analysed for K-ras codons 12 and 13 mutations by means of a combination of enriched polymerase chain reaction amplification and temporal temperature gradient electrophoresis. RESULTS—K-ras mutations were found in 21/161 (13%) microdissected samples in 7/13 large bowels (16 and five in codons 12 and 13, respectively), and in 10/100 (10%) mucosal DNA samples (six and four, respectively). One of four patients with six adenocarcinomas had a K-ras mutation in a carcinoma, as well as one of two patients with large dysplasia associated lesion or mass (DALM). Eight of 13 (61%) areas with villous architecture and large, distended goblet cells, had a K-ras mutation, which was significantly more frequent than in low grade dysplasia (one of 23, 4%) but did not reach significance versus high grade dysplasia (four of 14, 28.5%). K-ras mutations were found in one of 20 (5%) flat lesions indefinite for dysplasia, two of 14 (14%) in non-villous, hypermucinous mucosa, and in one of 57 flat areas negative for dysplasia. CONCLUSION—The highest K-ras mutation frequency was found in villous, hypermucinous mucosa. We suggest that this entity should be investigated further as a potential risk lesion for cancer development. It may represent a pathway directly from non-classical dysplasia to cancer, not previously described. Keywords: K-ras mutations; ulcerative colitis; dysplasia; dysplasia associated lesion or mass
Full Text
The Full Text of this article is available as a PDF (151.3 KB).
Figure 1 .
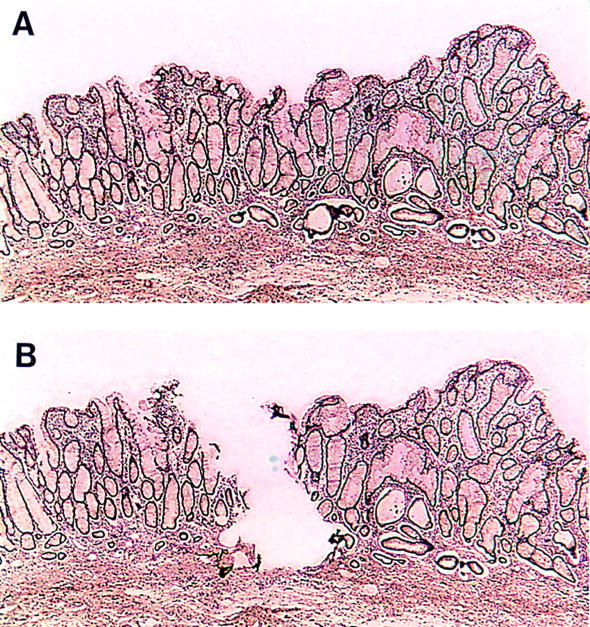
Section from large bowel mucosa of patient 2, rich in goblet cells, but without dysplasia, (A) before and (B) after microdissection.
Figure 2 .
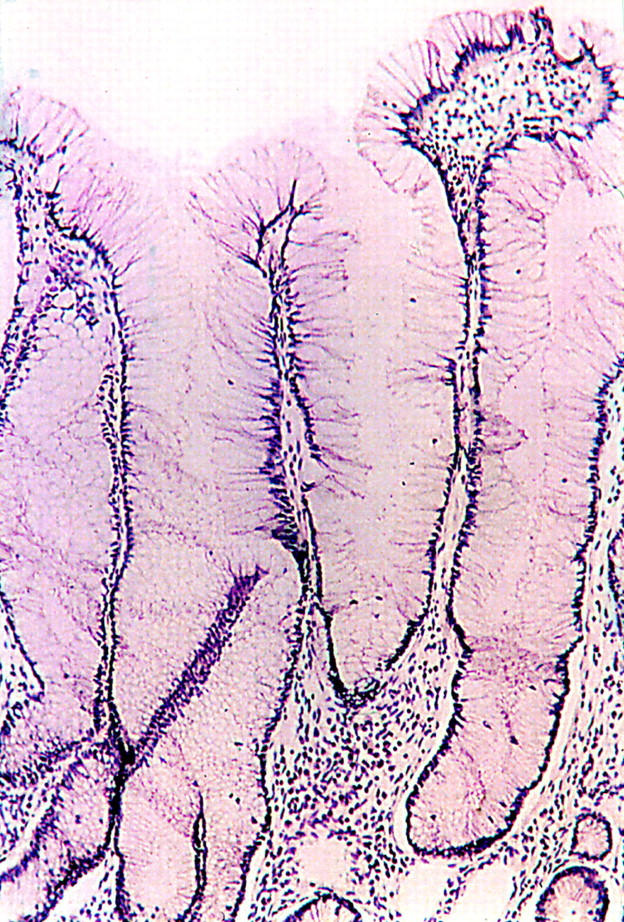
Mucosal specimen from patient 11, with villous growth pattern and elongated, distended goblet cells without cytological atypia. This area was K-ras mutation positive.
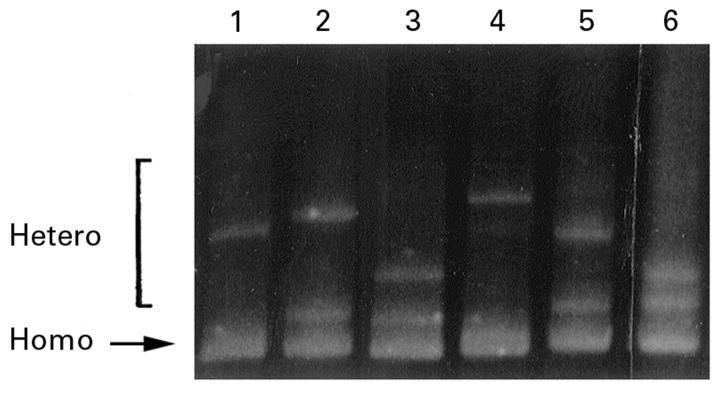
Figure 3 .
TTGE gel showing migration patterns of K-ras exon 1 fragments. All lanes show different migration patterns at the upper bands (heteroduplex bands), indicating different K-ras mutations. The lower band is the homoduplex band. Lanes 1-5 show codon 12 mutations: (1) GTT (valine); (2) TGT (cysteine); (3) GAT (aspartate); (4) GCT (alanine); and (5) AGT (serine). Lane 6 shows a codon 13 GAC mutation (aspartate).
Figure 4 .
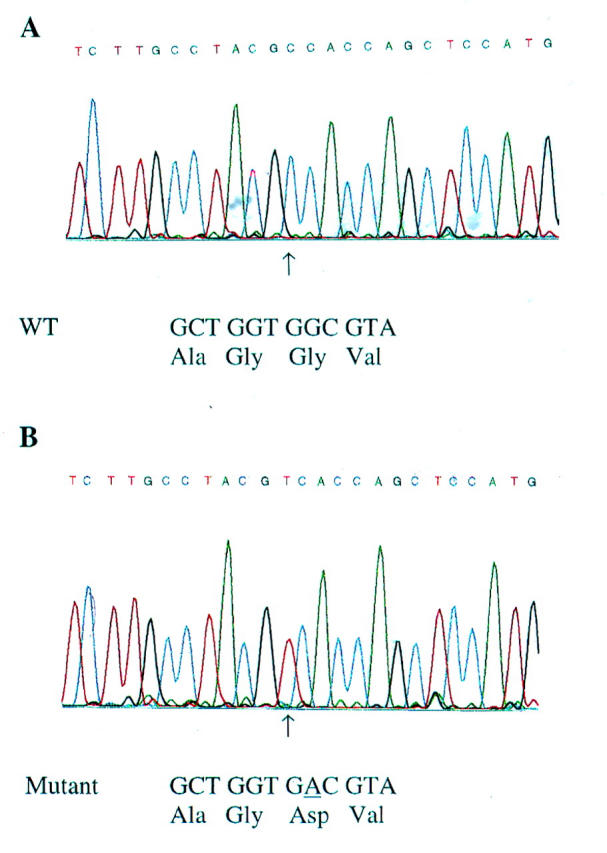
(A) Automated sequence analysis of K-ras, showing wild type sequence GGT in codon 12. This is an inverse analysis, and should be read from right to left. (B). K-ras mutation in the second base in codon 12, giving the sequence GAT (aspartate) instead of GGT (glycine).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen S. N., Løvig T., Breivik J., Lund E., Gaudernack G., Meling G. I., Rognum T. O. K-ras mutations and prognosis in large-bowel carcinomas. Scand J Gastroenterol. 1997 Jan;32(1):62–69. doi: 10.3109/00365529709025065. [DOI] [PubMed] [Google Scholar]
- Andreyev H. J., Norman A. R., Cunningham D., Oates J. R., Clarke P. A. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst. 1998 May 6;90(9):675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- Bell S. M., Kelly S. A., Hoyle J. A., Lewis F. A., Taylor G. R., Thompson H., Dixon M. F., Quirke P. c-Ki-ras gene mutations in dysplasia and carcinomas complicating ulcerative colitis. Br J Cancer. 1991 Jul;64(1):174–178. doi: 10.1038/bjc.1991.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhattar J., Losi L., Chaubert P., Givel J. C., Costa J. Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology. 1993 Apr;104(4):1044–1048. doi: 10.1016/0016-5085(93)90272-e. [DOI] [PubMed] [Google Scholar]
- Benhattar J., Saraga E. Molecular genetics of dysplasia in ulcerative colitis. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1171–1173. doi: 10.1016/0959-8049(95)00142-6. [DOI] [PubMed] [Google Scholar]
- Bjørheim J., Lystad S., Lindblom A., Kressner U., Westring S., Wahlberg S., Lindmark G., Gaudernack G., Ekstrøm P., Røe J. Mutation analyses of KRAS exon 1 comparing three different techniques: temporal temperature gradient electrophoresis, constant denaturant capillary electrophoresis and allele specific polymerase chain reaction. Mutat Res. 1998 Jul 17;403(1-2):103–112. doi: 10.1016/s0027-5107(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Burmer G. C., Levine D. S., Kulander B. G., Haggitt R. C., Rubin C. E., Rabinovitch P. S. c-Ki-ras mutations in chronic ulcerative colitis and sporadic colon carcinoma. Gastroenterology. 1990 Aug;99(2):416–420. doi: 10.1016/0016-5085(90)91024-z. [DOI] [PubMed] [Google Scholar]
- Chaubert P., Benhattar J., Saraga E., Costa J. K-ras mutations and p53 alterations in neoplastic and nonneoplastic lesions associated with longstanding ulcerative colitis. Am J Pathol. 1994 Apr;144(4):767–775. [PMC free article] [PubMed] [Google Scholar]
- Chen J., Compton C., Cheng E., Fromowitz F., Viola M. V. c-Ki-ras mutations in dysplastic fields and cancers in ulcerative colitis. Gastroenterology. 1992 Jun;102(6):1983–1987. doi: 10.1016/0016-5085(92)90323-q. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Greer C. E., Lund J. K., Manos M. M. PCR amplification from paraffin-embedded tissues: recommendations on fixatives for long-term storage and prospective studies. PCR Methods Appl. 1991 Aug;1(1):46–50. doi: 10.1101/gr.1.1.46. [DOI] [PubMed] [Google Scholar]
- Holzmann K., Klump B., Borchard F., Hsieh C. J., Kühn A., Gaco V., Gregor M., Porschen R. Comparative analysis of histology, DNA content, p53 and Ki-ras mutations in colectomy specimens with long-standing ulcerative colitis. Int J Cancer. 1998 Mar 30;76(1):1–6. doi: 10.1002/(sici)1097-0215(19980330)76:1<1::aid-ijc1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Iino H., Jass J. R., Simms L. A., Young J., Leggett B., Ajioka Y., Watanabe H. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999 Jan;52(1):5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S. E., Redston M., Seymour A. B., Caldas C., Powell S. M., Kornacki S., Kinzler K. W. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994 Aug;107(2):420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Lee R. G. Villous regeneration in ulcerative colitis. Arch Pathol Lab Med. 1987 Mar;111(3):276–278. [PubMed] [Google Scholar]
- McLellan E. A., Owen R. A., Stepniewska K. A., Sheffield J. P., Lemoine N. R. High frequency of K-ras mutations in sporadic colorectal adenomas. Gut. 1993 Mar;34(3):392–396. doi: 10.1136/gut.34.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer S. J., Mane S. M., Wood P. K., Resau J. H., Newkirk C., Terzakis J. A., Korelitz B. I., Weinstein W. M., Needleman S. W. Activation of c-Ki-ras in human gastrointestinal dysplasias determined by direct sequencing of polymerase chain reaction products. Cancer Res. 1990 Jun 15;50(12):3627–3630. [PubMed] [Google Scholar]
- Norheim Andersen S., Breivik J., Løvig T., Meling G. I., Gaudernack G., Clausen O. P., Schjölberg A., Fausa O., Langmark F., Lund E. K-ras mutations and HLA-DR expression in large bowel adenomas. Br J Cancer. 1996 Jul;74(1):99–108. doi: 10.1038/bjc.1996.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori K., Oda Y., Sugiyama K., Hasebe T., Mukai K., Fujii T., Tajiri H., Yoshida S., Fukushima S., Esumi H. High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut. 1997 May;40(5):660–663. doi: 10.1136/gut.40.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori K., Sugiyama K., Hasebe T., Fukushima S., Esumi H. Emergence of adenomatous aberrant crypt foci (ACF) from hyperplastic ACF with concomitant increase in cell proliferation. Cancer Res. 1995 Nov 1;55(21):4743–4746. [PubMed] [Google Scholar]
- Redston M. S., Papadopoulos N., Caldas C., Kinzler K. W., Kern S. E. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995 Feb;108(2):383–392. doi: 10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Symonds D. A., Vickery A. L. Mucinous carcinoma of the colon and rectum. Cancer. 1976 Apr;37(4):1891–1900. doi: 10.1002/1097-0142(197604)37:4<1891::aid-cncr2820370439>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Torres C., Antonioli D., Odze R. D. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow-up study of 89 polyps from 59 patients. Am J Surg Pathol. 1998 Mar;22(3):275–284. doi: 10.1097/00000478-199803000-00001. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Mochizuki M., Wakasa H. Detection of c-ras gene mutation and expression of p21 protein in dysplasias and carcinomas complicating ulcerative colitis. J Gastroenterol. 1995 Nov;30 (Suppl 8):30–32. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Williams G. T. Metaplastic (hyperplastic) polyps of the large bowel: benign neoplasms after all? Gut. 1997 May;40(5):691–692. doi: 10.1136/gut.40.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]