Abstract
BACKGROUND—Overexpression of regulatory peptide receptors in selected human tumours is of diagnostic and therapeutic relevance. AIMS—To evaluate the expression of somatostatin, vasoactive intestinal peptide (VIP), substance P, cholecystokinin (CCK) A and B, and neurotensin receptors in hepatocellular carcinoma (HCC). METHODS—In vitro receptor autoradiography for the various peptide receptors using selective iodinated radioligands on tissue sections in 59 cases of HCC. RESULTS—41% of HCC expressed somatostatin receptors; 47% expressed VIP receptors. VIP receptors were always identified in non-neoplastic liver tissue. Substance P receptors were only identified in 5% of HCC but in the majority of their peritumorous and intratumorous vessels. CCK-A and -B and neurotensin receptors were not detected in HCC. The somatostatin receptors showed high affinity for somatostatin and octreotide. The VIP receptors had high affinity for VIP, pituitary adenylate cyclase activating peptide (PACAP) 27, and a VIP1 selective analogue, suggesting the presence of VIP1/PACAP II type receptors. PACAP I receptors were identified in two cases. Substance P receptors were all of the NK1 subtype. The density of somatostatin receptors in HCC was low compared with the density found in liver metastases of neuroendocrine tumours. The VIP receptor density was always lower in HCC than in adjacent liver tissue. CONCLUSIONS—Somatostatin, VIP, and substance P may have a receptor mediated role in HCC. Substance P receptors may be involved in regulation of tumour associated blood flow; somatostatin receptors and VIP receptors may mediate tumour growth. Diagnostic and therapeutic evaluation of somatostatin and VIP analogues may be of interest in receptor positive HCC. Keywords: somatostatin receptors; vasoactive intestinal peptide receptors; substance P receptors; receptor autoradiography; tumour vasculature
Full Text
The Full Text of this article is available as a PDF (331.5 KB).
Figure 1 .
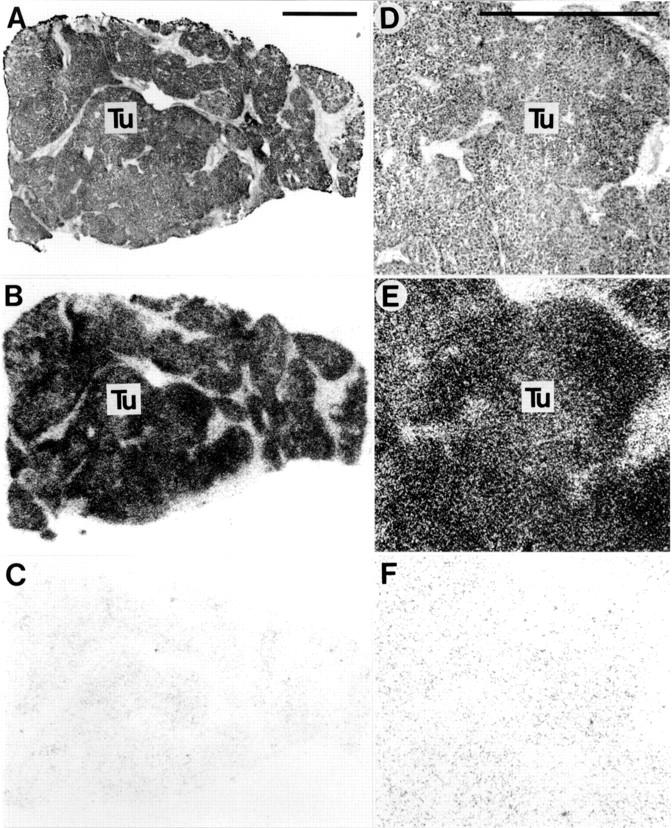
Somatostatin receptors in hepatocellular carcinoma. (A,D) Haematoxylin and eosin stained sections. D is an area of A at higher magnification. (B,E) Autoradiograms showing total binding of 125I-labelled [Tyr3]-octreotide. Homogenous labelling of tumour (Tu) tissue can be seen clearly. (C,F) Autoradiograms showing non-specific binding of 125I-labelled [Tyr3]-octreotide (in the presence of 10−6 M unlabelled octreotide). Bars = 1 mm.
Figure 2 .
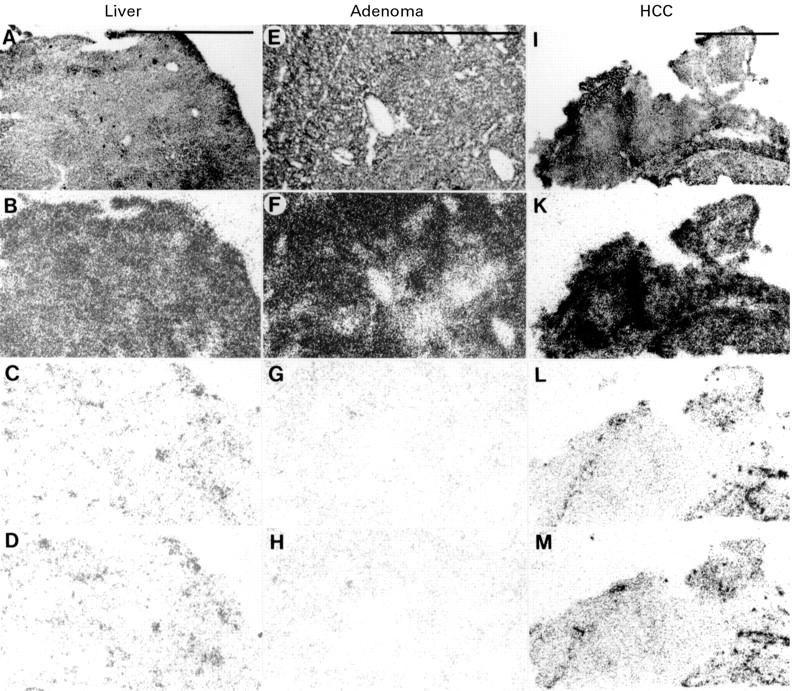
Vasoactive intestinal peptide (VIP) receptors in liver (A-D), hepatic adenoma (E-H), and hepatocellular carcinoma (HCC) (I-M). (A,E,I) haematoxylin and eosin stained sections. (B,F,K) Autoradiograms showing total binding of 125I-VIP. (C,G,L) Autoradiograms showing a discrete residual non-specific binding of 125I-VIP in the presence of 20 nM unlabelled VIP. (D,H,M) Autoradiograms showing the same residual non-specific binding of 125I-VIP in the presence of 20 nM pituitary adenylate cyclase activating peptide (PACAP) 27. All three tissues expressed VIP receptors. Bars = 1 mm.
Figure 3 .
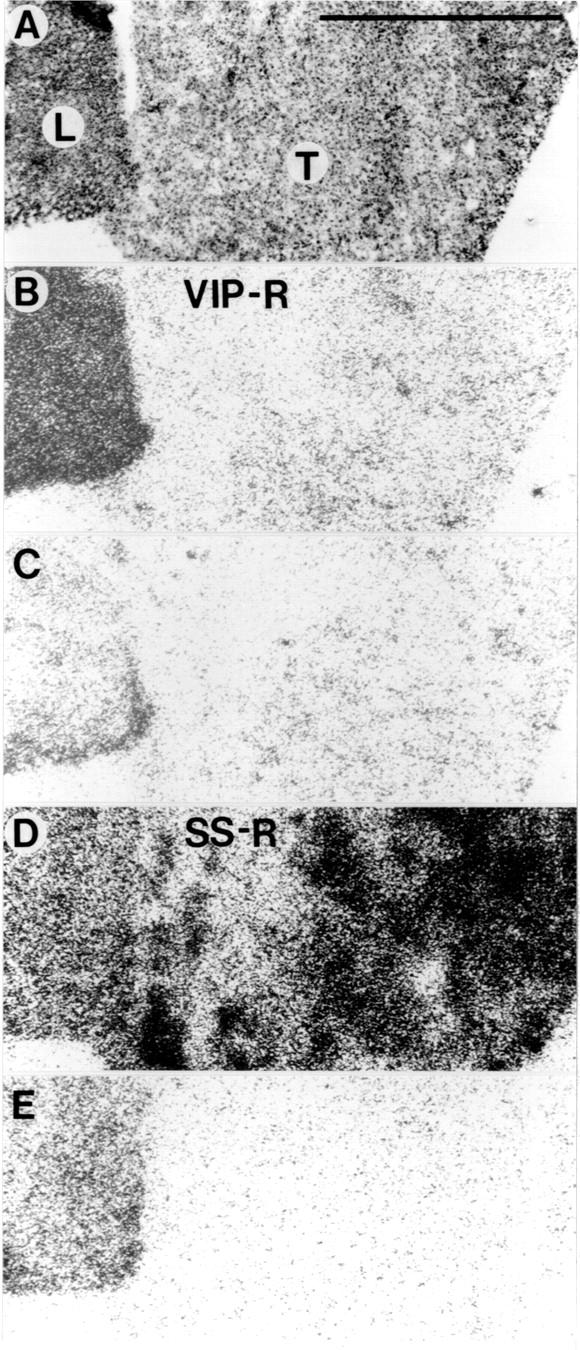
Vasoactive intestinal peptide (VIP) and somatostatin receptors in a surgically resected sample of hepatocellular carcinoma (HCC) containing tumour and liver tissue. (A) Haematoxylin and eosin stained section showing HCC (T) and adjacent liver (L). (B) Autoradiogram showing total binding of 125I-VIP in the section shown in A. (C) Autoradiogram showing non-specific binding of 125I-VIP. (D) Autoradiogram showing total binding of 125I-[Tyr3]-octreotide (corresponding to a section different but consecutive to that in A). (E) Autoradiogram showing non-specific binding of 125I-[Tyr3]-octreotide. VIP receptors were identified in liver tissue but not in HCC (B); somatostatin receptors were identified in HCC but not in liver (D). Liver had high non-specific binding for 125I-[Tyr3]-octreotide (seen in E). Bar = 1 mm.
Figure 4 .
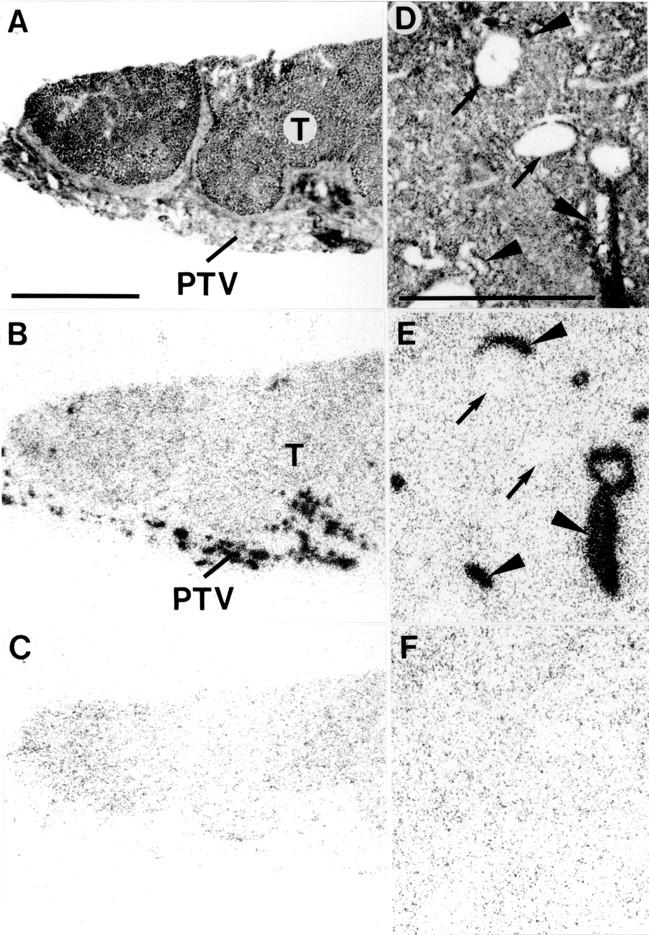
Substance P receptors in hepatocellular carcinoma (HCC) (A-C) and a hepatic adenoma (D-F). (A,D) Haematoxylin and eosin stained sections. In A, the tumour (T) is surrounded by stroma containing peritumorous vessels (PTV). In D, the adenoma contains arteries (arrowheads) and veins (arrows). (B,E) Autoradiograms showing total binding of 125I- BHSP (Bolton-Hunter substance P). (C,F) Autoradiograms showing non-specific binding of 125I- BHSP (in the presence of 10−6 M substance P). Peritumorous vessels are strongly labelled in B. Intratumorous arteries are strongly labelled in E, whereas veins are not. In both cases, the tumours are substance P receptor negative. Bars = 1 mm.
Figure 5 .
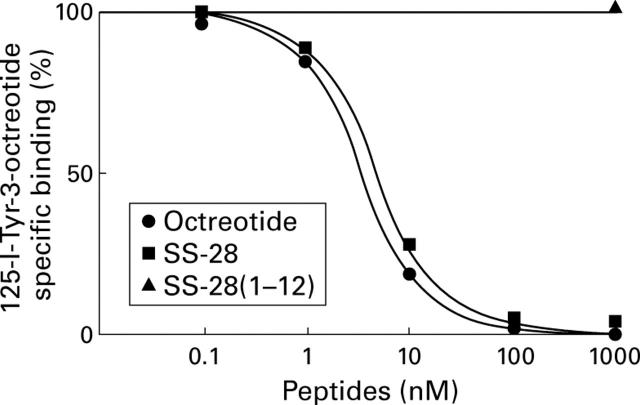
Displacement curve of 125I-labelled [Tyr3]-octreotide in tissue sections from hepatocellular carcinoma. Tissue sections were incubated with 65 pM 125I-labelled [Tyr3]-octreotide and increasing concentrations of unlabelled octreotide, somatostatin (SS) 28, or 1000 nM somatostatin 28(1-12). Each point represents the optical density of binding in a large, homogeneously labelled area of tumour tissue (n=4). Non-specific binding was subtracted from all values.
Figure 6 .
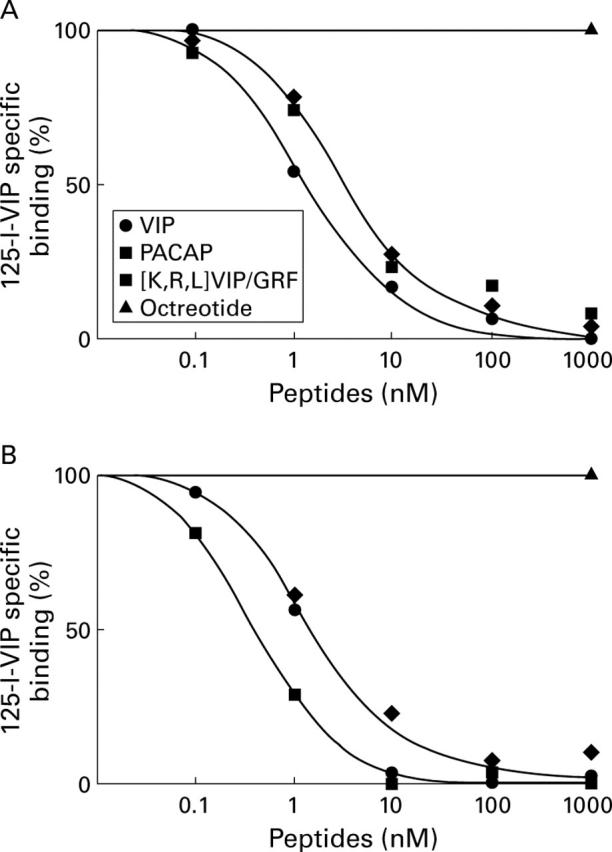
Displacement curves of specific 125I-vasoactive intestinal peptide (VIP) binding to tissue sections from (A) liver and (B) hepatocellular carcinoma. Tissue sections were incubated with 30 pM 125I-VIP and increasing concentrations of unlabelled VIP, pituitary adenylate cyclase activating peptide (PACAP) 27, [K15, R16, L17] VIP(1-7)/GRF(8-27), or 1000 nM of octreotide. Each point represents the optical density of binding in a large, homogeneously labelled area of liver or tumour tissue in one section. Non-specific binding was subtracted from all values.
Figure 7 .
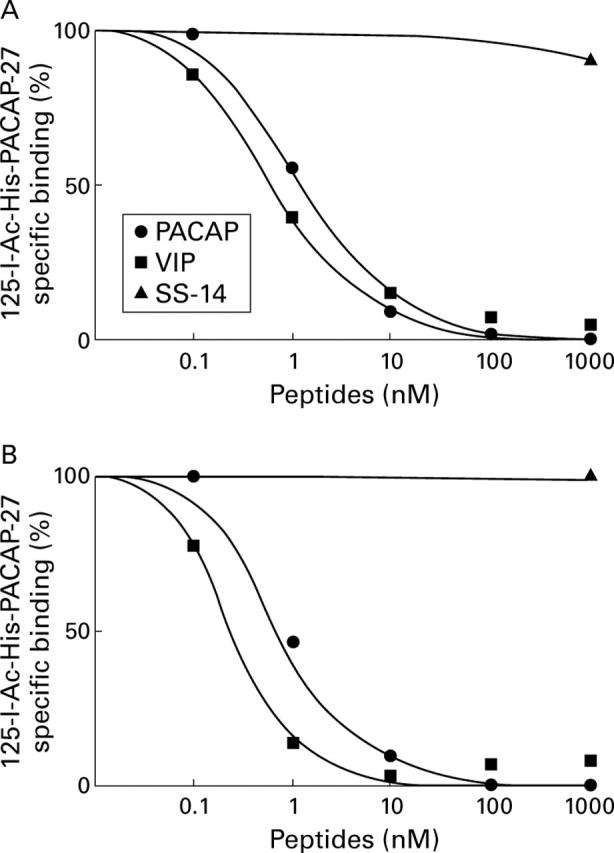
Displacement curves of specific 125I-N-acetyl-pituitary adenylate cyclase activating peptide (PACAP) 27 binding to tissue sections from (A) liver and (B) hepatocellular carcinoma. Tissue sections were incubated with 30 pM 125I-N-acetyl-PACAP-27 and increasing concentrations of unlabelled PACAP-27, vasoactive intestinal peptide (VIP), or 1000 nM of somatostatin. Each point represents the optical density of binding in a large, homogeneously labelled area of liver or tumour tissue in one section. Non-specific binding was subtracted from all values.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behr T. M., Jenner N., Radetzky S., Béhe M., Gratz S., Yücekent S., Raue F., Becker W. Targeting of cholecystokinin-B/gastrin receptors in vivo: preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur J Nucl Med. 1998 Apr;25(4):424–430. doi: 10.1007/s002590050241. [DOI] [PubMed] [Google Scholar]
- Collier N. A., Weinbren K., Bloom S. R., Lee Y. C., Hodgson H. J., Blumgart L. H. Neurotensin secretion by fibrolamellar carcinoma of the liver. Lancet. 1984 Mar 10;1(8376):538–540. doi: 10.1016/s0140-6736(84)90934-6. [DOI] [PubMed] [Google Scholar]
- EDMONDSON H. A., STEINER P. E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954 May;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gourlet P., Vandermeers A., Vertongen P., Rathe J., De Neef P., Cnudde J., Waelbroeck M., Robberecht P. Development of high affinity selective VIP1 receptor agonists. Peptides. 1997;18(10):1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- Hennig I. M., Laissue J. A., Horisberger U., Reubi J. C. Substance-P receptors in human primary neoplasms: tumoral and vascular localization. Int J Cancer. 1995 Jun 9;61(6):786–792. doi: 10.1002/ijc.2910610608. [DOI] [PubMed] [Google Scholar]
- Kouroumalis E., Skordilis P., Thermos K., Vasilaki A., Moschandrea J., Manousos O. N. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998 Mar;42(3):442–447. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts S. W., Krenning E. P., Reubi J. C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991 Nov;12(4):450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- Otte A., Mueller-Brand J., Dellas S., Nitzsche E. U., Herrmann R., Maecke H. R. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet. 1998 Feb 7;351(9100):417–418. doi: 10.1016/s0140-6736(05)78355-0. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Levivier M., Cadet J. L. Neurotensin receptors in human meningiomas. Ann Neurol. 1991 Nov;30(5):650–654. doi: 10.1002/ana.410300504. [DOI] [PubMed] [Google Scholar]
- Reubi J. C. In vitro identification of vasoactive intestinal peptide receptors in human tumors: implications for tumor imaging. J Nucl Med. 1995 Oct;36(10):1846–1853. [PubMed] [Google Scholar]
- Reubi J. C., Kvols L. K., Waser B., Nagorney D. M., Heitz P. U., Charboneau J. W., Reading C. C., Moertel C. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990 Sep 15;50(18):5969–5977. [PubMed] [Google Scholar]
- Reubi J. C. Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med. 1995 Oct;36(10):1825–1835. [PubMed] [Google Scholar]
- Reubi J. C. New specific radioligand for one subpopulation of brain somatostatin receptors. Life Sci. 1985 May 13;36(19):1829–1836. doi: 10.1016/0024-3205(85)90155-9. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Perrin M. H., Rivier J. E., Vale W. High affinity binding sites for a somatostatin-28 analog in rat brain. Life Sci. 1981 May 11;28(19):2191–2198. doi: 10.1016/0024-3205(81)90628-7. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Schaer J. C., Waser B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997 Apr 1;57(7):1377–1386. [PubMed] [Google Scholar]
- Reubi J. C., Waser B., Friess H., Büchler M., Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut. 1998 Apr;42(4):546–550. doi: 10.1136/gut.42.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Waser B., Läderach U., Stettler C., Friess H., Halter F., Schmassmann A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997 Apr;112(4):1197–1205. doi: 10.1016/s0016-5085(97)70131-8. [DOI] [PubMed] [Google Scholar]
- Schindel D. T., Grosfeld J. L. Hepatic resection enhances growth of residual intrahepatic and subcutaneous hepatoma, which is inhibited by octreotide. J Pediatr Surg. 1997 Jul;32(7):995–998. doi: 10.1016/s0022-3468(97)90385-7. [DOI] [PubMed] [Google Scholar]
- Virgolini I., Raderer M., Kurtaran A., Angelberger P., Banyai S., Yang Q., Li S., Banyai M., Pidlich J., Niederle B. Vasoactive intestinal peptide-receptor imaging for the localization of intestinal adenocarcinomas and endocrine tumors. N Engl J Med. 1994 Oct 27;331(17):1116–1121. doi: 10.1056/NEJM199410273311703. [DOI] [PubMed] [Google Scholar]
- Zhao M., Laissue J. A., Zimmermann A. "Neuroendocrine" differentiation in hepatocellular carcinomas (HCCs): immunohistochemical reactivity is related to distinct tumor cell types, but not to tumor grade. Histol Histopathol. 1993 Oct;8(4):617–626. [PubMed] [Google Scholar]