Abstract
METHODS—Four patients with clinicopathological features suggesting a new distinct entity defining extensive small intestinal CD4 T cell infiltration were observed. RESULTS—All four patients presented with chronic diarrhoea, malabsorption, and weight loss. Biopsy specimens of the small intestine disclosed extensive and diffuse infiltration of the lamina propria by pleomorphic small T lymphocytes, which were positive for CD3, CD4, CD5, and the β chain of T cell receptor in all three cases studied and negative for CD103 in all three cases studied. It is notable that, in all invaded areas, the infiltrating cells showed no histological change throughout the whole evolution. In three patients, lymphocyte proliferation was monoclonal and there was extraintestinal involvement. In one patient, lymphoproliferation was oligoclonal and confined to the small intestine. In all four patients, there was no evidence of coeliac disease. Although none of the four patients responded to single or multiple drug chemotherapy, median survival was five years. CONCLUSION—Extensive small intestinal CD4 T cell infiltration is a rare entity, distinct from coeliac disease and associated with prolonged survival. Keywords: CD4; T cells; lymphocytes; small intestine
Full Text
The Full Text of this article is available as a PDF (223.7 KB).
Figure 1 .
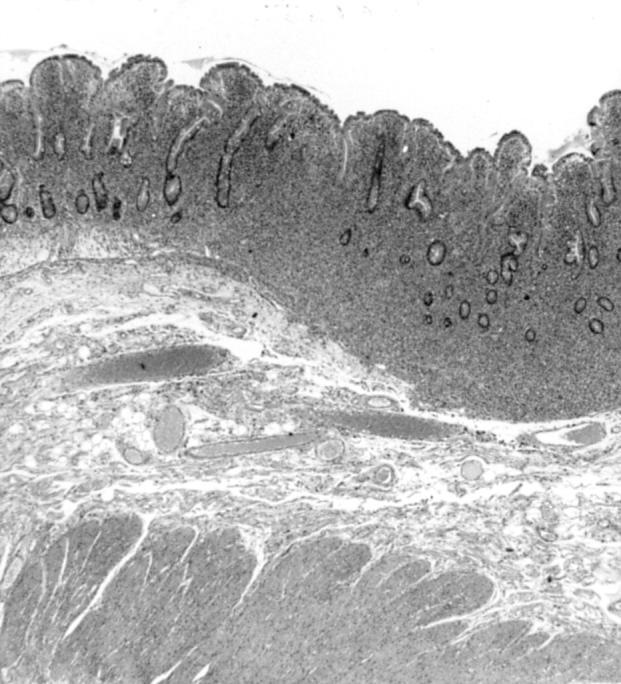
Surgical jejunal resection showing dense lymphoid infiltrate of mucosa and superficial part of submucosa and some degree of villous atrophy. There is no evidence of coeliac disease (patient 1). Haematoxylin-eosin G stain; original magnification × 75.
Figure 2 .
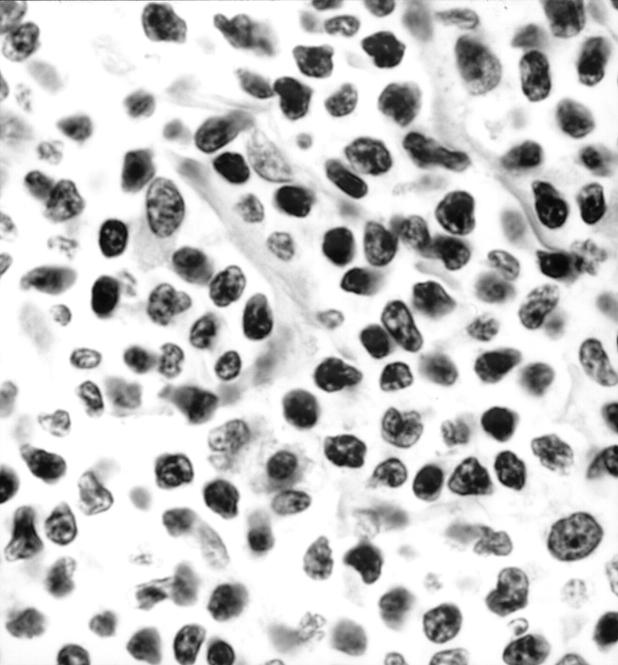
Surgical jejunal resection at a higher magnification, in which tumour cells appear as small pleomorphic cells with irregularly shaped indented nuclei (patient 2). Haematoxylin-eosin stain; original magnification × 3000.
Figure 3 .
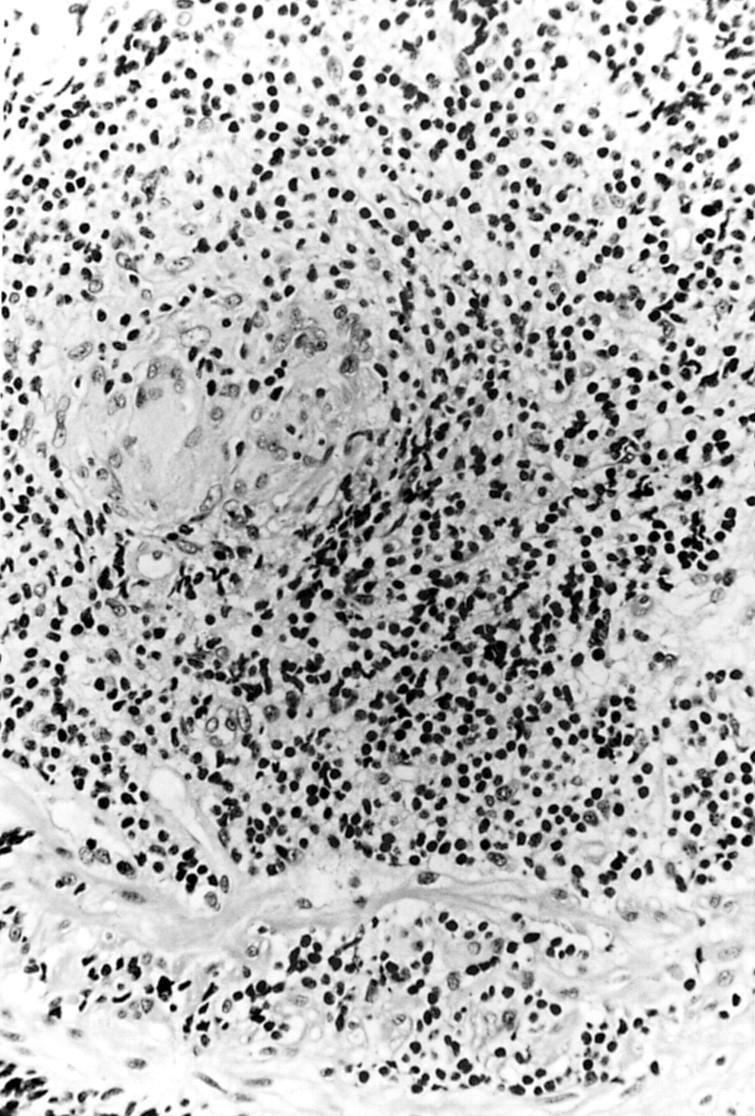
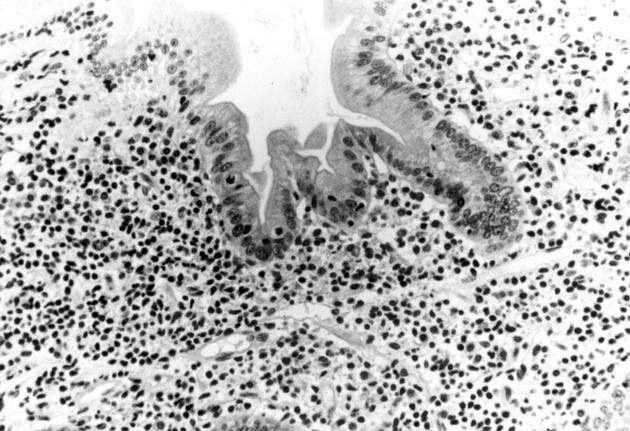
Surgical jejunal resection from patient 2. Mucosal pleomorphic small T cell infiltrate, associated with a non-caseating granuloma, and extending into the muscularis mucosa. Haematoxylin-eosin stain; original magnification × 750.
Figure 4 .
Surgical jejunal resection from patient 3. Mucosal pleomorphic small T cell infiltrate is not associated with intraepithelial lymphocytosis. Enterocytes appear normal. Haematoxylin-eosin stain; original magnification × 750.
Figure 5 .
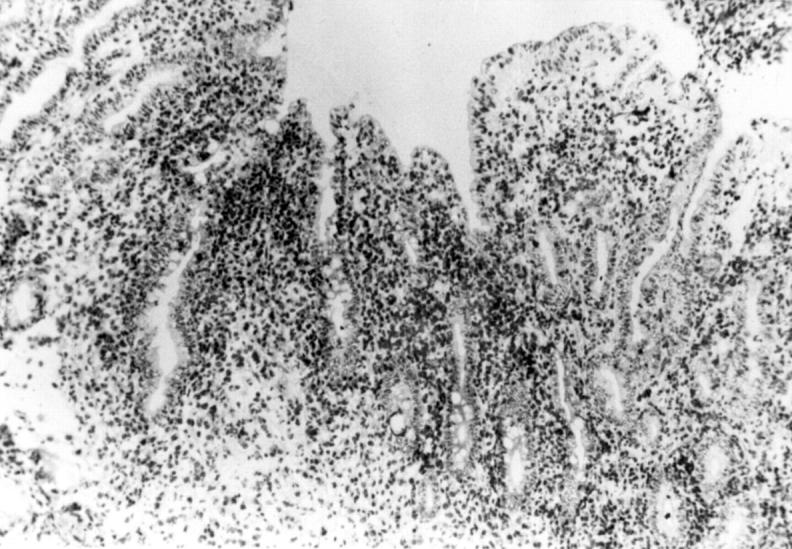
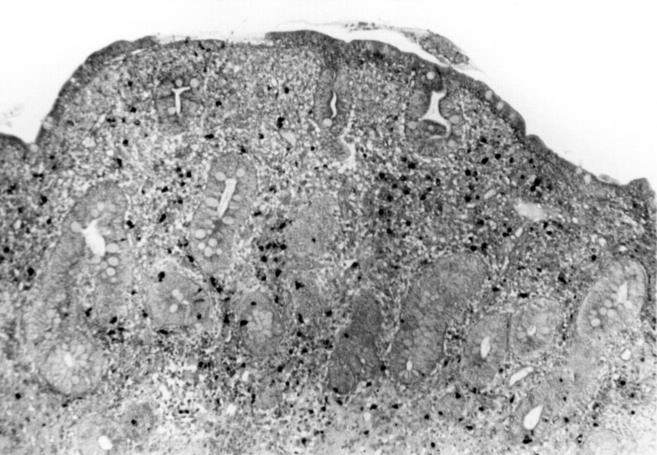
Immunohistochemical examination of a frozen jejunal biopsy specimen from patient 4 showing a dense intramucosal CD4+ T cell infiltrate. Anti-CD4 antibody-diaminobenzidine-peroxidase stain; original magnification × 300.
Figure 6 .
In situ hybridization on duodenojejunal biopsy specimen from patient 4 showing total villous atrophy and positivity of some cells within the lamina propria. EBER probe; original magnification × 300.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfsen G. C., Beiske K., Bell H., Marton P. F. Low-grade intestinal lymphoma of intraepithelial T lymphocytes with concomitant enteropathy-associated T cell lymphoma: case report suggesting a possible histogenetic relationship. Hum Pathol. 1989 Sep;20(9):909–913. doi: 10.1016/0046-8177(89)90105-6. [DOI] [PubMed] [Google Scholar]
- Ashton-Key M., Diss T. C., Pan L., Du M. Q., Isaacson P. G. Molecular analysis of T-cell clonality in ulcerative jejunitis and enteropathy-associated T-cell lymphoma. Am J Pathol. 1997 Aug;151(2):493–498. [PMC free article] [PubMed] [Google Scholar]
- Bachelez H., Bioul L., Flageul B., Baccard M., Moulonguet-Michau I., Verola O., Morel P., Dubertret L., Sigaux F. Detection of clonal T-cell receptor gamma gene rearrangements with the use of the polymerase chain reaction in cutaneous lesions of mycosis fungoides and Sézary syndrome. Arch Dermatol. 1995 Sep;131(9):1027–1031. [PubMed] [Google Scholar]
- Carbonnel F., Grollet-Bioul L., Brouet J. C., Teilhac M. F., Cosnes J., Angonin R., Deschaseaux M., Châtelet F. P., Gendre J. P., Sigaux F. Are complicated forms of celiac disease cryptic T-cell lymphomas? Blood. 1998 Nov 15;92(10):3879–3886. [PubMed] [Google Scholar]
- Carbonnel F., Lavergne A., Messing B., Tsapis A., Berger R., Galian A., Nemeth J., Brouet J. C., Rambaud J. C. Extensive small intestinal T-cell lymphoma of low-grade malignancy associated with a new chromosomal translocation. Cancer. 1994 Feb 15;73(4):1286–1291. doi: 10.1002/1097-0142(19940215)73:4<1286::aid-cncr2820730425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cellier C., Patey N., Mauvieux L., Jabri B., Delabesse E., Cervoni J. P., Burtin M. L., Guy-Grand D., Bouhnik Y., Modigliani R. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998 Mar;114(3):471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- Chiu E. K., Loke S. L., Chan A. C., Liang R. H. T-lymphoblastic lymphoma arising in the small intestine. Pathology. 1991 Oct;23(4):356–359. doi: 10.3109/00313029109063605. [DOI] [PubMed] [Google Scholar]
- Chott A., Dragosics B., Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. Am J Pathol. 1992 Dec;141(6):1361–1371. [PMC free article] [PubMed] [Google Scholar]
- Cooper B. T., Read A. E. Coeliac disease and lymphoma. Q J Med. 1987 Apr;63(240):269–274. [PubMed] [Google Scholar]
- Domizio P., Owen R. A., Shepherd N. A., Talbot I. C., Norton A. J. Primary lymphoma of the small intestine. A clinicopathological study of 119 cases. Am J Surg Pathol. 1993 May;17(5):429–442. doi: 10.1097/00000478-199305000-00001. [DOI] [PubMed] [Google Scholar]
- Egan L. J., Walsh S. V., Stevens F. M., Connolly C. E., Egan E. L., McCarthy C. F. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol. 1995 Sep;21(2):123–129. [PubMed] [Google Scholar]
- Foucar K., Foucar E., Mitros F., Clamon G., Goeken J., Crossett J. Epitheliotropic lymphoma of the small bowel. Report of a fatal case with cytotoxic/suppressor T-cell immunotype. Cancer. 1984 Jul 1;54(1):54–60. doi: 10.1002/1097-0142(19840701)54:1<54::aid-cncr2820540113>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Freeman C., Berg J. W., Cutler S. J. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972 Jan;29(1):252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gras M. P., Laâbi Y., Linares-Cruz G., Blondel M. O., Rigaut J. P., Brouet J. C., Leca G., Haguenauer-Tsapis R., Tsapis A. BCMAp: an integral membrane protein in the Golgi apparatus of human mature B lymphocytes. Int Immunol. 1995 Jul;7(7):1093–1106. doi: 10.1093/intimm/7.7.1093. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Magidson J. G., Weiss L. M., Hu E., Warnke H. A., Lewin K. J. Gastrointestinal lymphomas. Immunohistochemical studies on the cell of origin. Am J Surg Pathol. 1985 May;9(5):328–337. [PubMed] [Google Scholar]
- Hattori T., Asou N., Suzushima H., Takatsuki K., Tanaka K., Naito K., Natori H., Oizumi K. Leukaemia of novel gastrointestinal T-lymphocyte population infected with HTLV-I. Lancet. 1991 Jan 12;337(8733):76–77. doi: 10.1016/0140-6736(91)90737-a. [DOI] [PubMed] [Google Scholar]
- Hirakawa K., Fuchigami T., Nakamura S., Daimaru Y., Ohshima K., Sakai Y., Ichimaru T. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology. 1996 Sep;111(3):778–782. doi: 10.1053/gast.1996.v111.pm8780585. [DOI] [PubMed] [Google Scholar]
- Hsiao C. H., Lee W. I., Chang S. L., Su I. J. Angiocentric T-cell lymphoma of the intestine: a distinct etiology of ischemic bowel disease. Gastroenterology. 1996 Apr;110(4):985–990. doi: 10.1053/gast.1996.v110.pm8613032. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., O'Connor N. T., Spencer J., Bevan D. H., Connolly C. E., Kirkham N., Pollock D. J., Wainscoat J. S., Stein H., Mason D. Y. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985 Sep 28;2(8457):688–691. doi: 10.1016/s0140-6736(85)92930-7. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Wright D. H. Malignant histiocytosis of the intestine. Its relationship to malabsorption and ulcerative jejunitis. Hum Pathol. 1978 Nov;9(6):661–677. doi: 10.1016/s0046-8177(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Kanavaros P., Lavergne A., Galian A., Boivin P., Fourmestreaux A. R., Priollet B. C., Flandrin G., Hautefeuille P. A primary immunoblastic T malignant lymphoma of the small bowel, with azurophilic intracytoplasmic granules. A histologic, immunologic, and electron microscopy study. Am J Surg Pathol. 1988 Aug;12(8):641–647. [PubMed] [Google Scholar]
- Laabi Y., Gras M. P., Brouet J. C., Berger R., Larsen C. J., Tsapis A. The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res. 1994 Apr 11;22(7):1147–1154. doi: 10.1093/nar/22.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne A., Brochériou I., Delfau M. H., Copie-Bergman C., Houdart R., Gaulard P. H. Primary intestinal gamma-delta T-cell lymphoma with evidence of Epstein-Barr virus. Histopathology. 1998 Mar;32(3):271–276. doi: 10.1046/j.1365-2559.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- Laâbi Y., Gras M. P., Carbonnel F., Brouet J. C., Berger R., Larsen C. J., Tsapis A. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992 Nov;11(11):3897–3904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughran T. P., Jr, Kadin M. E., Deeg H. J. T-cell intestinal lymphoma associated with celiac sprue. Ann Intern Med. 1986 Jan;104(1):44–47. doi: 10.7326/0003-4819-104-1-44. [DOI] [PubMed] [Google Scholar]
- Macintyre E. A., d'Auriol L., Duparc N., Leverger G., Galibert F., Sigaux F. Use of oligonucleotide probes directed against T cell antigen receptor gamma delta variable-(diversity)-joining junctional sequences as a general method for detecting minimal residual disease in acute lymphoblastic leukemias. J Clin Invest. 1990 Dec;86(6):2125–2135. doi: 10.1172/JCI114951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Touchard G., Lemaire M., Babin P., Demeocq F., Fonck Y., Meyer M., Preud'Homme J. L. Malignant lymphoma of the small bowel associated with diffuse nodular lymphoid hyperplasia. N Engl J Med. 1985 Jul 18;313(3):166–171. doi: 10.1056/NEJM198507183130307. [DOI] [PubMed] [Google Scholar]
- Murray A., Cuevas E. C., Jones D. B., Wright D. H. Study of the immunohistochemistry and T cell clonality of enteropathy-associated T cell lymphoma. Am J Pathol. 1995 Feb;146(2):509–519. [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Suchi T., Koshikawa T., Takagi N., Hayashi K., Koike K., Suzuki H., Ogura M., Kurita S., Oyama A. Aggressive rectal lymphoma of large granular lymphocytes with the histologic feature of an angiocentric growth pattern. Cancer. 1993 Jan 1;71(1):249–256. doi: 10.1002/1097-0142(19930101)71:1<249::aid-cncr2820710138>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pan L., Diss T. C., Peng H., Lu Q., Wotherspoon A. C., Thomas J. A., Isaacson P. G. Epstein-Barr virus (EBV) in enteropathy-associated T-cell lymphoma (EATL). J Pathol. 1993 Jun;170(2):137–143. doi: 10.1002/path.1711700207. [DOI] [PubMed] [Google Scholar]
- Radaszkiewicz T., Dragosics B., Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992 May;102(5):1628–1638. doi: 10.1016/0016-5085(92)91723-h. [DOI] [PubMed] [Google Scholar]
- Rambaud J. C., Halphen M., Galian A., Tsapis A. Immunoproliferative small intestinal disease (IPSID): relationships with alpha-chain disease and "Mediterranean" lymphomas. Springer Semin Immunopathol. 1990;12(2-3):239–250. doi: 10.1007/BF00197509. [DOI] [PubMed] [Google Scholar]
- Ruskoné-Fourmestraux A., Aegerter P., Delmer A., Brousse N., Galian A., Rambaud J. C. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d'Etude des Lymphomes Digestifs. Gastroenterology. 1993 Dec;105(6):1662–1671. doi: 10.1016/0016-5085(93)91061-l. [DOI] [PubMed] [Google Scholar]
- Salles G., Herbrecht R., Tilly H., Berger F., Brousse N., Gisselbrecht C., Coiffier B. Aggressive primary gastrointestinal lymphomas: review of 91 patients treated with the LNH-84 regimen. A study of the Groupe d'Etude des Lymphomes Agressifs. Am J Med. 1991 Jan;90(1):77–84. doi: 10.1016/0002-9343(91)90509-v. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Hummel M., Zemlin M., Schneider T., Ullrich R., Heise W., Zeitz M., Riecken E. O., Stein H. Intestinal T-cell lymphoma: a reassessment of cytomorphological and phenotypic features in relation to patterns of small bowel remodelling. Virchows Arch. 1996 Sep;429(1):27–36. doi: 10.1007/BF00196817. [DOI] [PubMed] [Google Scholar]
- Shepherd N. A., Blackshaw A. J., Hall P. A., Bostad L., Coates P. J., Lowe D. G., Levison D. A., Morson B. C., Stansfeld A. G. Malignant lymphoma with eosinophilia of the gastrointestinal tract. Histopathology. 1987 Feb;11(2):115–130. doi: 10.1111/j.1365-2559.1987.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Tallini G., West A. B., Buckley P. J. Diagnosis of gastrointestinal T-cell lymphomas in routinely processed tissues. J Clin Gastroenterol. 1993 Jul;17(1):57–66. doi: 10.1097/00004836-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Tokunaga O., Watanabe T., Shimamoto Y., Tokudome S. Primary T-cell lymphoma of the gastrointestinal tract associated with human T-cell lymphotropic virus type I. An analysis using in situ hybridization and polymerase chain reaction. Cancer. 1993 Feb 1;71(3):708–716. doi: 10.1002/1097-0142(19930201)71:3<708::aid-cncr2820710309>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Tsapis A., Bentaboulet M., Pellet P., Mihaesco E., Thierry D., Seligmann M., Brouet J. C. The productive gene for alpha-H chain disease protein MAL is highly modified by insertion-deletion processes. J Immunol. 1989 Dec 1;143(11):3821–3827. [PubMed] [Google Scholar]
- Wright D. H., Jones D. B., Clark H., Mead G. M., Hodges E., Howell W. M. Is adult-onset coeliac disease due to a low-grade lymphoma of intraepithelial T lymphocytes? Lancet. 1991 Jun 8;337(8754):1373–1374. doi: 10.1016/0140-6736(91)93059-i. [DOI] [PubMed] [Google Scholar]
- de Bruin P. C., Jiwa N. M., Oudejans J. J., Radaszkiewicz T., Meijer C. J. Epstein-Barr virus in primary gastrointestinal T cell lymphomas. Association with gluten-sensitive enteropathy, pathological features, and immunophenotype. Am J Pathol. 1995 Apr;146(4):861–867. [PMC free article] [PubMed] [Google Scholar]