Abstract
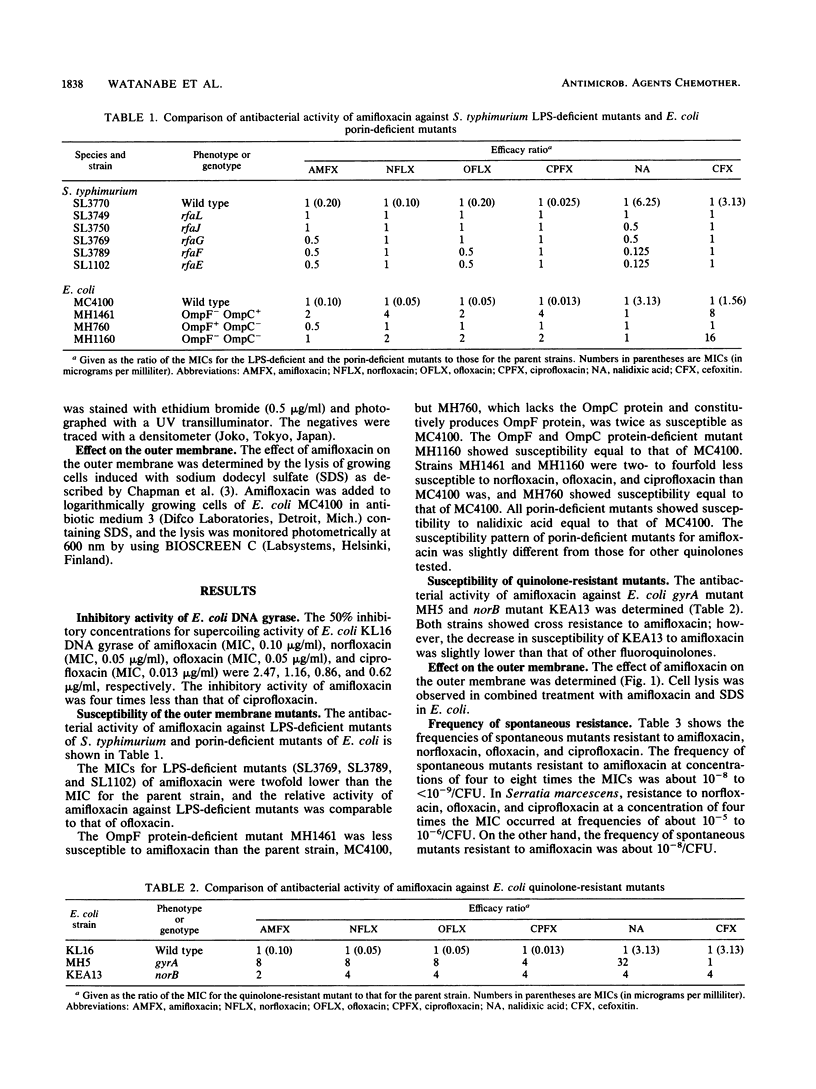
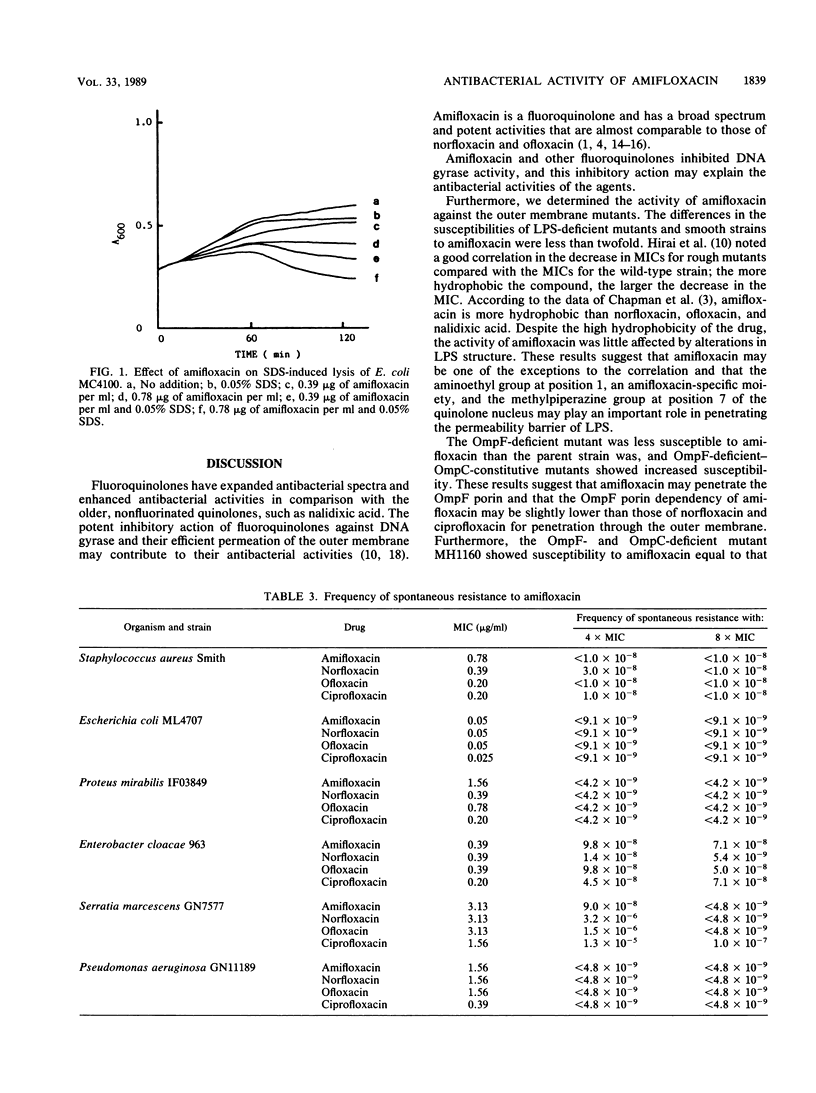
Amifloxacin showed potent inhibitory activity against DNA gyrase of Escherichia coli. The difference in the susceptibilities of lipopolysaccharide-deficient Salmonella typhimurium mutants and their parent strain was less than twofold, and the difference in the susceptibilities of porin-deficient E. coli mutants and their parent strain was less than twofold. There was cross resistance among the quinolone group of agents; however, the decrease in MIC for norB mutants was slightly lower than that of other fluoroquinolones. Cell lysis was induced with combined treatment of amifloxacin and sodium dodecyl sulfate in E. coli. The frequency of mutants spontaneously resistant to amifloxacin was extremely low in all species tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassey C. M., Baltch A. L., Smith R. P. Comparative antimicrobial activity of enoxacin, ciprofloxacin, amifloxacin, norfloxacin and ofloxacin against 177 bacterial isolates. J Antimicrob Chemother. 1986 May;17(5):623–628. doi: 10.1093/jac/17.5.623. [DOI] [PubMed] [Google Scholar]
- Chapman J. S., Bertasso A., Georgopapadakou N. H. Fleroxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):239–241. doi: 10.1128/aac.33.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Wagner R. B., Dobson R. A., Wentland M. P., Bailey D. M. In vitro and in vivo antibacterial activities of the fluoroquinolone WIN 49375 (amifloxacin). Antimicrob Agents Chemother. 1985 Jan;27(1):4–10. doi: 10.1128/aac.27.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Fisher L. M., O'Dea M. H. DNA gyrase: purification and catalytic properties of a fragment of gyrase B protein. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6289–6293. doi: 10.1073/pnas.76.12.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Okamoto R., Inoue M., Mitsuhashi S. Effects of beta-lactamases and omp mutation on susceptibility to beta-lactam antibiotics in Escherichia coli. Antimicrob Agents Chemother. 1989 Mar;33(3):382–386. doi: 10.1128/aac.33.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani A., Welty G. S., Newton B. R., Richard G. A. Effects of changes in pH, medium, and inoculum size on the in vitro activity of amifloxacin against urinary isolates of Staphylococcus saprophyticus and Escherichia coli. Antimicrob Agents Chemother. 1985 Apr;27(4):449–451. doi: 10.1128/aac.27.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus N. V., Tally F. P., Barza M. Antimicrobial spectrum of Win 49375. Antimicrob Agents Chemother. 1984 Jul;26(1):104–107. doi: 10.1128/aac.26.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. F., Jr, Twitty J. A. Amifloxacin activity against well-defined gentamicin-resistant, gram-negative bacteria. Antimicrob Agents Chemother. 1984 Nov;26(5):781–784. doi: 10.1128/aac.26.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouno K., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activity of AT-2266. Antimicrob Agents Chemother. 1983 Jul;24(1):78–84. doi: 10.1128/aac.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F., Inokuchi K., Matsuyama S., Mizushima S. Mutation causing reverse osmoregulation of synthesis of OmpF, a major outer membrane protein of Escherichia coli. J Bacteriol. 1984 Aug;159(2):688–692. doi: 10.1128/jb.159.2.688-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]


