Abstract
BACKGROUND—A previous study showed that the glucocorticoid dexamethasone, at doses of 100 µg/kg and above, inhibited leucocyte adhesion to rat mesenteric postcapillary venules activated with interleukin 1β (IL-1β), as assessed by videomicroscopy. AIMS—To identify whether the adhesion molecule, intercellular adhesion molecule 1 (ICAM-1), or the chemokine KC could be targeted by the steroid to mediate its antiadhesive effect. METHODS—Rat mesenteries were treated with IL-1β (20 ng intraperitoneally) and the extent of leucocyte adhesion measured at two and four hours using intravital microscopy. Rats were treated with dexamethasone, and passively immunised against ICAM-1 or KC. Endogenous expression of these two mediators was validated by immunohistochemistry, ELISA, and the injection of specific radiolabelled antibodies. RESULTS—Dexamethasone greatly reduced IL-1β induced leucocyte adhesion, endothelial expression of ICAM-1 in the postcapillary venule, and release of the mast cell derived chemokine KC. Injection of specific antibodies to the latter mediators was also extremely effective in downregulating (>80%) IL-1β induced leucocyte adhesion. CONCLUSIONS—Induction by IL-1β of endogenous ICAM-1 and KC contributes to leucocyte adhesion to inflamed mesenteric vessels. Without excluding other possible mediators, these data clearly show that dexamethasone interferes with ICAM-1 expression and KC release from mast cells, resulting in suppression of leucocyte accumulation in the bowel wall, which is a prominent feature of several gastrointestinal pathologies. Keywords: inflammation; glucocorticoids; intravital microscopy; mast cell; neutrophil; endothelium
Full Text
The Full Text of this article is available as a PDF (162.6 KB).
Figure 1 .
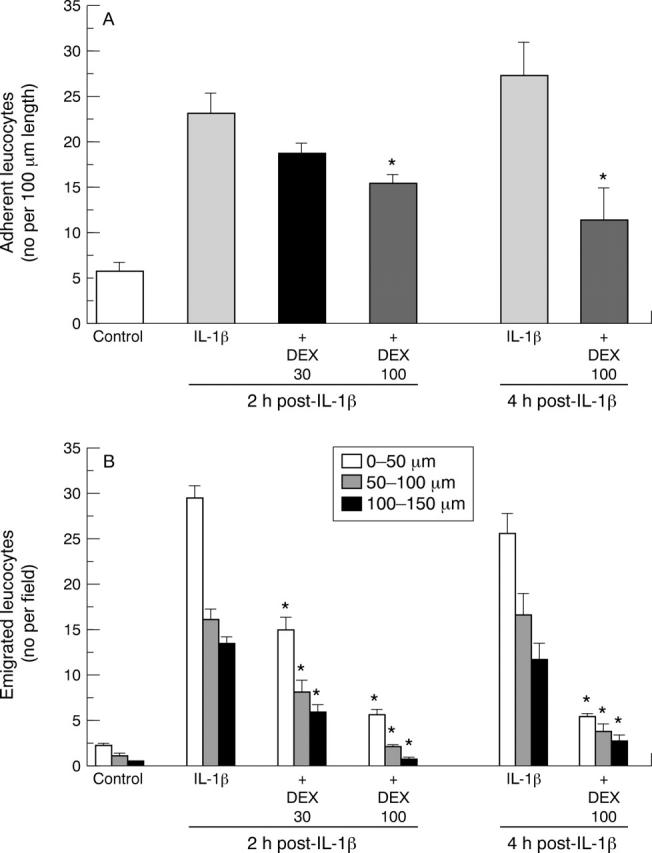
Effect of dexamethasone (DEX) on interleukin (IL) 1β induced leucocyte adhesion and emigration in rat mesenteric postcapillary venules. Data are mean (SEM); n=6 rats. *p<0.05 v respective IL-1β group.
Figure 2 .
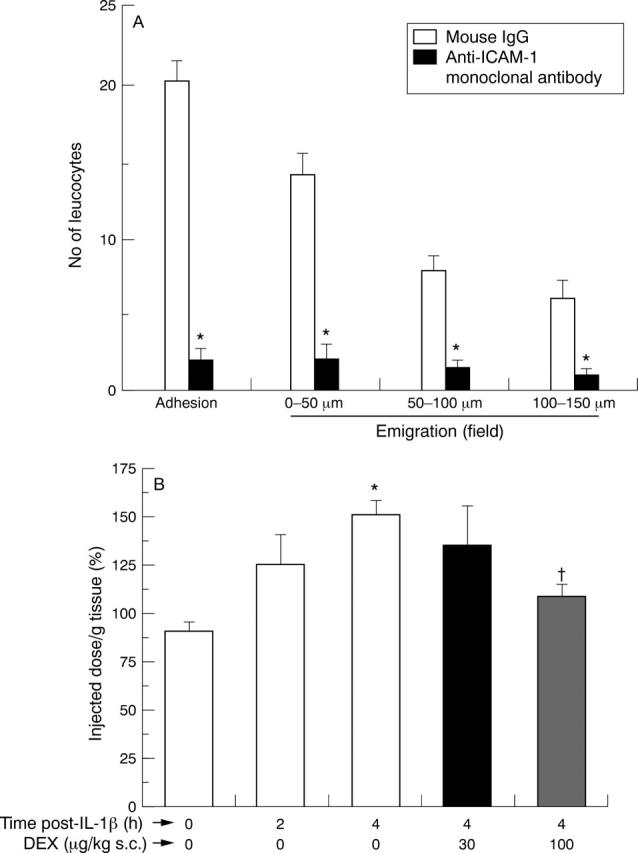
Involvement of intercellular adhesion molecule (ICAM) 1 in interleukin (IL) 1β induced cell adhesion and emigration. (A) Animals were treated with 2 mg/kg mouse IgG or mouse antirat ICAM-1 monoclonal antibody one hour prior to IL-1β administration (20 ng intraperitoneally). Mesenteries were exposed two hours later, and the degree of cell adhesion (per 100 µm vessel wall) and emigration quantified by videomicroscopy. Data are mean (SEM); n=6 rats. *p<0.05 v mouse IgG group. (B) ICAM-1 expression in the mesenteric tissue as assessed by the dual antibody technique. Rats were treated with rat IL-1β (20 ng intraperitoneally) and some of them pretreated with dexamethasone (DEX) one hour prior to the cytokine. At the reported times post-IL-1β, the specific radioactivity due to endogenous ICAM-1 was determined. Data are mean (SEM); n=5 rats per group. *p<0.05 v control group (time 0); †p<0.05 v IL-1β group (time 4 h).
Figure 3 .
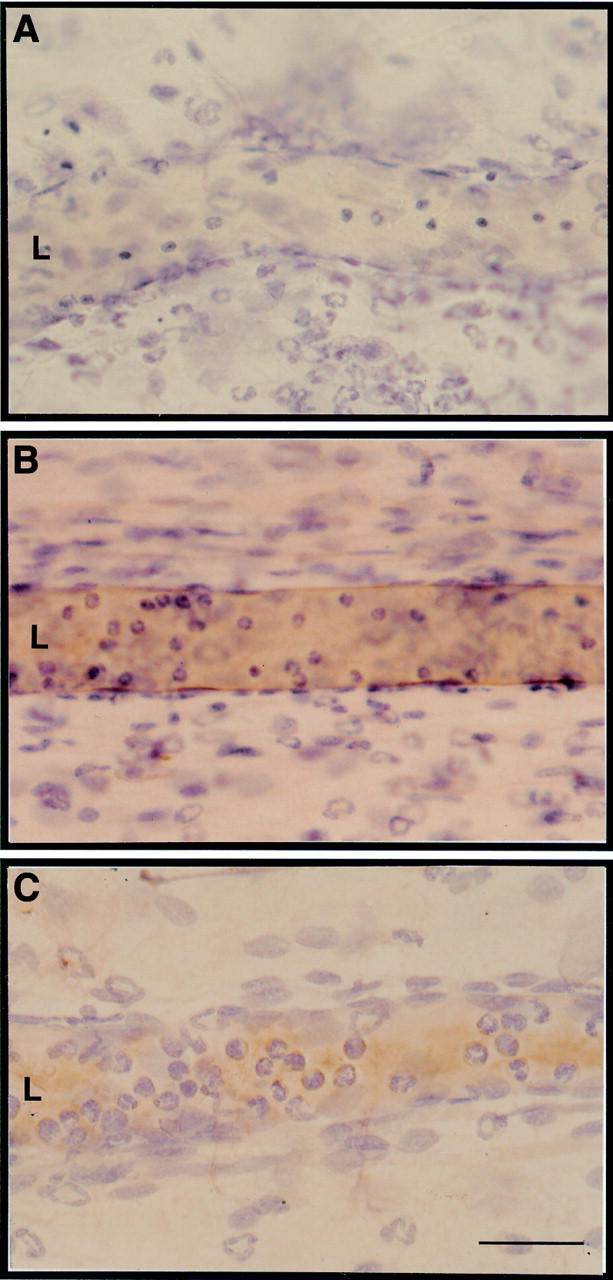
Localisation of intercellular adhesion molecule (ICAM) 1 immunostaining in inflamed rat mesenteric postcapillary venules. Typical micrographs of the ileal section of rat mesentery whole mounts. (A) Preparations excised two hours post-administration of 20 ng rat interleukin (IL) 1β from an animal injected with control mouse IgG 10 minutes prior to sacrifice. (B) As for A, but the rat was injected with 2 mg/kg mouse antirat ICAM-1 10 minutes prior to sacrifice and tissue collection. Note the brown staining around the endothelium of the postcapillary venule. (C) As in B, but the rat was treated with 100 µg/kg subcutaneous dexamethasone one hour prior to intraperitoneal injection of IL-1β. Note the scarce brown immunostaining compared with B. Pictures are representative of five distinct preparations. L, vessel lumen. Bar, 30 µm.
Figure 4 .
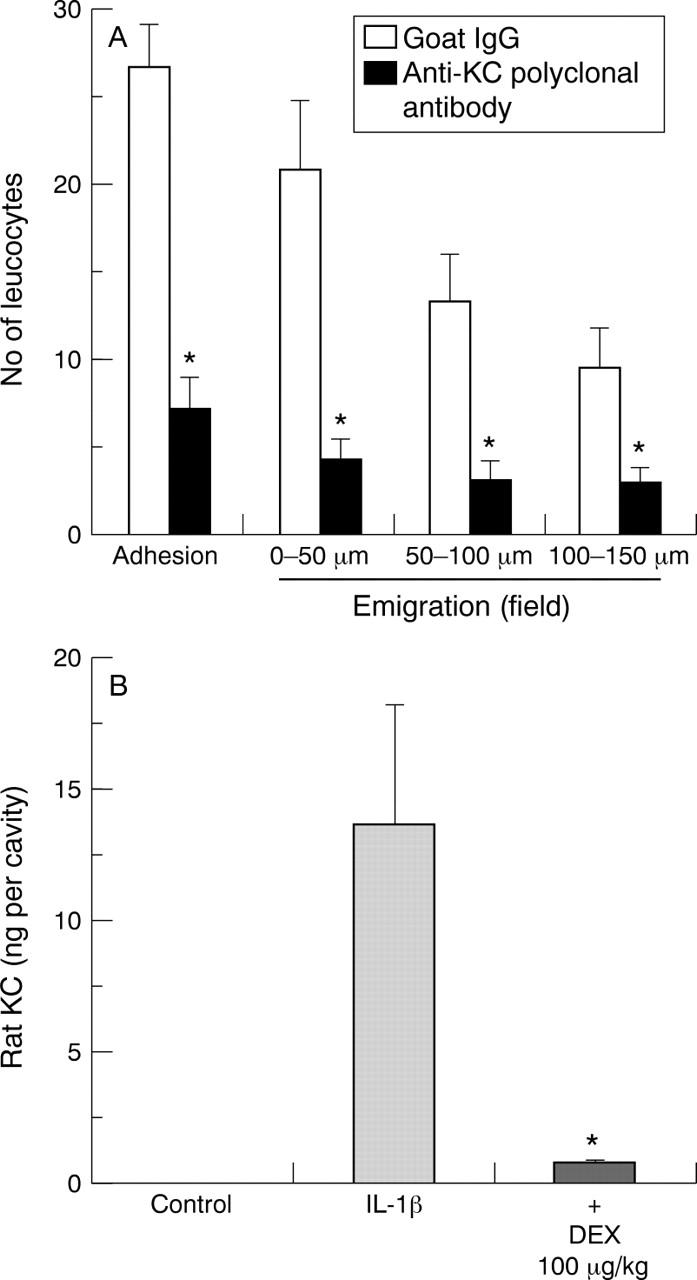
Involvement of KC in interleukin (IL) 1β induced cell adhesion and emigration. (A) Animals were treated with 2 mg goat IgG or goat antirat rat KC polyclonal antibody one hour prior to IL-1β administration (20 ng intraperitoneally). Mesenteries were exposed two hours later, and the degree of cell adhesion (per 100 µm vessel wall) and emigration quantified by videomicroscopy. Data are mean (SEM); n=8 rats. *p<0.05 v mouse IgG group. (B) Detection of rat KC protein in the peritoneal cell free lavage fluids of control rats (n=4), rats injected with IL-1β (20 ng intraperitoneally; n=5), and animals pretreated with dexamethasone (DEX) one hour prior to IL-1β administration (n=5). Lavage fluids were collected two hours post-IL-1β. Data are mean (SEM). *p<0.05 v IL-1β group.
Figure 5 .
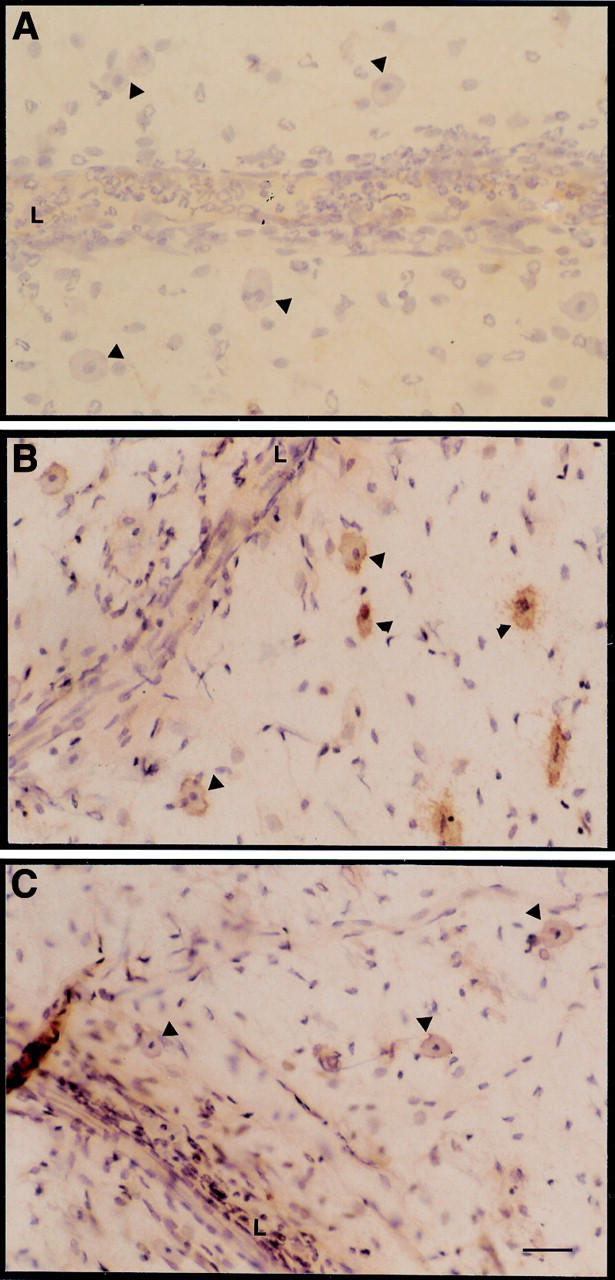
Localisation of KC immunostaining in inflamed rat mesenteric postcapillary venules. Typical micrographs of the ileal section of rat mesentery whole mounts. (A) Preparations excised two hours after administration of 20 ng rat interleukin (IL) 1β and incubated with non-specific rabbit IgG. Arrowheads indicate some of the perivenular mast cells. (B) As in A, but the tissue was incubated with specific rabbit antirat KC IgG. Arrowheads indicate some of the perivenular mast cells which stained for KC. (C) As in B, but the rat was treated with 100 µg/kg subcutaneous dexamethasone one hour prior to intraperitoneal injection of IL-1β. Arrowheads indicate some of the perivenular mast cells which appeared less stained for KC when compared with B. Pictures are representative of four distinct preparations. L, vessel lumen. Bar, 25 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickston S. J., Cominelli F. Inflammatory bowel disease: short- and long-term treatments. Adv Intern Med. 1998;43:143–174. [PubMed] [Google Scholar]
- Burns A. R., Walker D. C., Brown E. S., Thurmon L. T., Bowden R. A., Keese C. R., Simon S. I., Entman M. L., Smith C. W. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997 Sep 15;159(6):2893–2903. [PubMed] [Google Scholar]
- Caldenhoven E., Liden J., Wissink S., Van de Stolpe A., Raaijmakers J., Koenderman L., Okret S., Gustafsson J. A., Van der Saag P. T. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995 Apr;9(4):401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Clark B. D., Schindler R., Lierena R., Eysselein V. E., Thompson R. C., Dinarello C. A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990 Sep;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Frevert C. W., Huang S., Danaee H., Paulauskis J. D., Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995 Jan 1;154(1):335–344. [PubMed] [Google Scholar]
- Granger D. N., Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994 May;55(5):662–675. [PubMed] [Google Scholar]
- Henninger D. D., Panés J., Eppihimer M., Russell J., Gerritsen M., Anderson D. C., Granger D. N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997 Feb 15;158(4):1825–1832. [PubMed] [Google Scholar]
- Komatsu S., Flores S., Gerritsen M. E., Anderson D. C., Granger D. N. Differential up-regulation of circulating soluble and endothelial cell intercellular adhesion molecule-1 in mice. Am J Pathol. 1997 Jul;151(1):205–214. [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc Res. 1996 Oct;32(4):699–708. [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Luster A. D. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998 Feb 12;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Mancuso F., Flower R. J., Perretti M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule. Involvement of endogenous lipocortin 1. J Immunol. 1995 Jul 1;155(1):377–386. [PubMed] [Google Scholar]
- Möller A., Lippert U., Lessmann D., Kolde G., Hamann K., Welker P., Schadendorf D., Rosenbach T., Luger T., Czarnetzki B. M. Human mast cells produce IL-8. J Immunol. 1993 Sep 15;151(6):3261–3266. [PubMed] [Google Scholar]
- Nakagawa H., Ikesue A., Hatakeyama S., Kato H., Gotoda T., Komorita N., Watanabe K., Miyai H. Production of an interleukin-8-like chemokine by cytokine-stimulated rat NRK-49F fibroblasts and its suppression by anti-inflammatory steroids. Biochem Pharmacol. 1993 Apr 6;45(7):1425–1430. doi: 10.1016/0006-2952(93)90041-t. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Miyai H., Hirata M., Watanabe K., Onuma I. Synergism between interleukin-1 beta and tumor necrosis factor-alpha in production by 3T3 cells of a chemotactic factor for rat polymorphonuclear leukocytes. Inflammation. 1989 Oct;13(5):553–559. doi: 10.1007/BF00916761. [DOI] [PubMed] [Google Scholar]
- O'Leary E. C., Marder P., Zuckerman S. H. Glucocorticoid effects in an endotoxin-induced rat pulmonary inflammation model: differential effects on neutrophil influx, integrin expression, and inflammatory mediators. Am J Respir Cell Mol Biol. 1996 Jul;15(1):97–106. doi: 10.1165/ajrcmb.15.1.8679228. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T., Kubota A., Hirano T., Watanabe K., Yoshida H., Tsurufuji M., Iizuka Y., Konishi K., Tsurufuji S. Glucocorticoid-mediated gene suppression of rat cytokine-induced neutrophil chemoattractant CINC/gro, a member of the interleukin-8 family, through impairment of NF-kappa B activation. J Biol Chem. 1996 Jan 19;271(3):1651–1659. doi: 10.1074/jbc.271.3.1651. [DOI] [PubMed] [Google Scholar]
- Panés J., Granger D. N. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998 May;114(5):1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- Panés J., Perry M. A., Anderson D. C., Manning A., Leone B., Cepinskas G., Rosenbloom C. L., Miyasaka M., Kvietys P. R., Granger D. N. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995 Dec;269(6 Pt 2):H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- Perretti M., Croxtall J. D., Wheller S. K., Goulding N. J., Hannon R., Flower R. J. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996 Nov;2(11):1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci. 1997 Nov;18(11):418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Schleimer R. P. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45 (Suppl 1):S3–S44. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- Selvan R. S., Butterfield J. H., Krangel M. S. Expression of multiple chemokine genes by a human mast cell leukemia. J Biol Chem. 1994 May 13;269(19):13893–13898. [PubMed] [Google Scholar]
- Suzuki H., Zweifach B. W., Forrest M. J., Schmid-Schönbein G. W. Modification of leukocyte adhesion in spontaneously hypertensive rats by adrenal corticosteroids. J Leukoc Biol. 1995 Jan;57(1):20–26. [PubMed] [Google Scholar]
- Tailor A., Das A. M., Getting S. J., Flower R. J., Perretti M. Subacute treatment of rats with dexamethasone reduces ICAM-1 levels on circulating monocytes. Biochem Biophys Res Commun. 1997 Feb 24;231(3):675–678. doi: 10.1006/bbrc.1997.6168. [DOI] [PubMed] [Google Scholar]
- Tailor A., Flower R. J., Perretti M. Dexamethasone inhibits leukocyte emigration in rat mesenteric post-capillary venules: an intravital microscopy study. J Leukoc Biol. 1997 Sep;62(3):301–308. doi: 10.1002/jlb.62.3.301. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kitamura F., Kuida K., Shirao M., Mochizuki M., Suematsu M., Schmid-Schönbein G. W., Watanabe K., Tsurufuji S., Miyasaka M. Characterization of rat LECAM-1 (L-selectin) by the use of monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur J Immunol. 1993 Sep;23(9):2181–2188. doi: 10.1002/eji.1830230920. [DOI] [PubMed] [Google Scholar]
- Von Andrian U. H., Hansell P., Chambers J. D., Berger E. M., Torres Filho I., Butcher E. C., Arfors K. E. L-selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol. 1992 Oct;263(4 Pt 2):H1034–H1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- Wakelin M. W., Sanz M. J., Dewar A., Albelda S. M., Larkin S. W., Boughton-Smith N., Williams T. J., Nourshargh S. An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J Exp Med. 1996 Jul 1;184(1):229–239. doi: 10.1084/jem.184.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Fujioka M., Yokokawa H., Konishi K., Tsurufuji S., Nakagawa H. Rat gro/melanoma growth-stimulating activity. Assessment of the structure responsible for chemotactic activity by use of its fragments prepared by proteolysis and chemical synthesis. Cytokine. 1992 Jan;4(1):12–17. doi: 10.1016/1043-4666(92)90030-u. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Watanabe K., Suematsu M., Iida M., Takaishi K., Iizuka Y., Suzuki H., Suzuki M., Tsuchiya M., Tsurufuji S. Effect of rat CINC/gro, a member of the interleukin-8 family, on leukocytes in microcirculation of the rat mesentery. Exp Mol Pathol. 1992 Feb;56(1):60–69. doi: 10.1016/0014-4800(92)90023-5. [DOI] [PubMed] [Google Scholar]
- Yi E. S., Remick D. G., Lim Y., Tang W., Nadzienko C. E., Bedoya A., Yin S., Ulich T. R. The intratracheal administration of endotoxin: X. Dexamethasone downregulates neutrophil emigration and cytokine expression in vivo. Inflammation. 1996 Apr;20(2):165–175. doi: 10.1007/BF01487403. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Yoshikawa T., Nakamura Y., Takenaka S., Sakamoto K., Manabe H., Nakagawa S., Kondo M. Methylprednisolone inhibits neutrophil-endothelial cell interactions induced by interleukin-1beta under flow conditions. Life Sci. 1997;60(25):2341–2347. doi: 10.1016/s0024-3205(97)00290-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Oppenheim J. J., Leonard E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987 Aug 1;139(3):788–793. [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- Zagorski J., Wahl S. M. Inhibition of acute peritoneal inflammation in rats by a cytokine-induced neutrophil chemoattractant receptor antagonist. J Immunol. 1997 Aug 1;159(3):1059–1062. [PubMed] [Google Scholar]
- Zimmerman B. J., Holt J. W., Paulson J. C., Anderson D. C., Miyasaka M., Tamatani T., Todd R. F., 3rd, Rusche J. R., Granger D. N. Molecular determinants of lipid mediator-induced leukocyte adherence and emigration in rat mesenteric venules. Am J Physiol. 1994 Mar;266(3 Pt 2):H847–H853. doi: 10.1152/ajpheart.1994.266.3.H847. [DOI] [PubMed] [Google Scholar]


