Abstract
BACKGROUND—Altered expression of lamins A/C and B1, constituent proteins of the nuclear lamina, may occur during differentiation and has also been reported in primary lung cancer. AIMS—To examine the expression of these proteins in gastrointestinal neoplasms. PATIENTS—Archival human paraffin wax blocks and frozen tissue from patients undergoing surgical resection or endoscopic biopsy. METHODS—Immunohistochemistry and western blotting using polyclonal antisera against A type lamins and lamin B1. RESULTS—The expression of lamin A/C was reduced and was frequently undetectable by immunohistochemistry in all primary colon carcinomas and adenomas, and in 7/8 primary gastric cancers. Lamin B1 expression was reduced in all colon cancers, 16/18 colonic adenomas, and 6/8 gastric cancers. Aberrant, cytoplasmic labelling with both antibodies occurred in some colonic cancers and around one third of colonic adenomas. Cytoplasmic lamin A/C expression was detected in 3/8 gastric cancers. Lamin expression was reduced in gastric dysplasia, but not intestinal metaplasia, atrophy, or chronic gastritis. Lamin expression was low in carcinomas of oesophagus, prostate, breast, and uterus, but not pancreas. CONCLUSIONS—Reduced expression of nuclear lamins, sometimes together with aberrant, cytoplasmic immunoreactivity is common in gastrointestinal neoplasms. Altered lamin expression may be a biomarker of malignancy in the gastrointestinal tract. Keywords: nuclear lamins; biomarkers; gastrointestinal neoplasms
Full Text
The Full Text of this article is available as a PDF (237.4 KB).
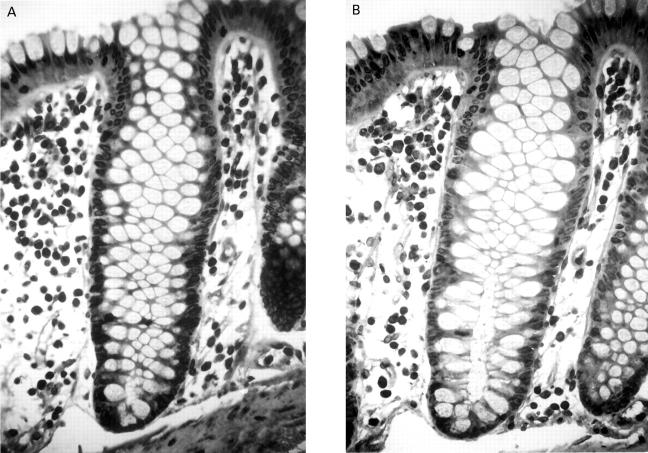
Figure 1 .
Normal colonic crypt immunostained for lamin A/C (A) and lamin B1 (B) showing dark nuclear membrane staining in both epithelial cells and lamina propria cells. Lamin A/C is most highly expressed in cells in the upper crypt. Original magnification × 400.
Figure 2 .
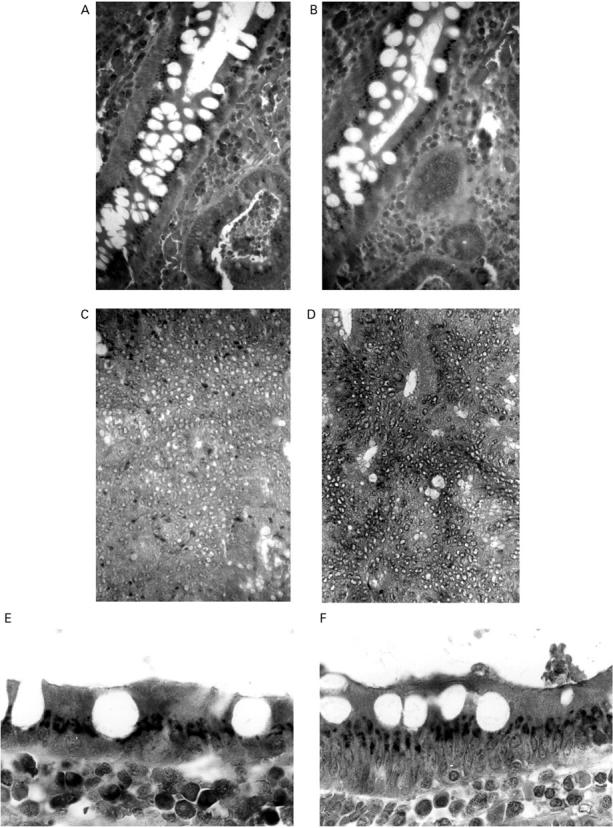
Expression of lamins in colon neoplasms. There is absent nuclear immunoreactivity with antibodies against lamin A/C (A) and B1 (B), and cytoplasmic labelling of lamin A/C (A) and B1 (B) in the gland of an adenomatous colonic polyp. In a colonic adenocarcinoma, lamin A/C is absent (C) and lamin B1 notably reduced (D). Original magnification × 400. The cytoplasmic immunolabelling of lamin A/C (E) and lamin B1 (F) between the nucleus and apical pole of adenomatous epithelial cells is better appreciated at higher magnification (× 1000).
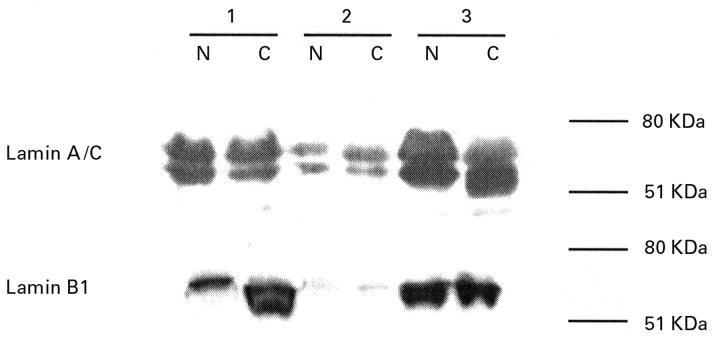
Figure 3 .
Representative immunoblot of normal (N) and cancer (C) tissue pairs from three patients with primary colorectal carcinoma. No consistent differences in the expression of lamin A/C or B1 were found in these tissue lysates. Migration of molecular mass standards in kilodaltons is shown on the right.
Figure 4 .
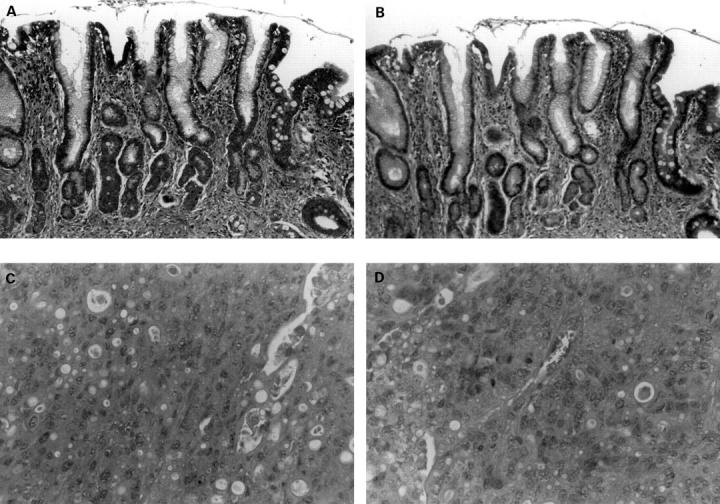
Expression of nuclear lamins in gastric tissue. In the non-neoplastic mucosa from a gastrectomy specimen removed for cancer, strong expression of nuclear lamin A/C (A) and B1 (B) is evident throughout, including both the relatively normal mucosa on the left and in the area of intestinal metaplasia, on the right. There is notably reduced expression of lamin A/C (C) and B1 (D) in an intestinal type gastric cancer. Original magnification × 400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Biamonti G., Giacca M., Perini G., Contreas G., Zentilin L., Weighardt F., Guerra M., Della Valle G., Saccone S., Riva S. The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol Cell Biol. 1992 Aug;12(8):3499–3506. doi: 10.1128/mcb.12.8.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broers J. L., Raymond Y., Rot M. K., Kuijpers H., Wagenaar S. S., Ramaekers F. C. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am J Pathol. 1993 Jul;143(1):211–220. [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N., Courvalin J. C. Stepwise reassembly of the nuclear envelope at the end of mitosis. J Cell Biol. 1993 Jul;122(2):295–306. doi: 10.1083/jcb.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N., McMahon C., Blobel G. Primary structure of a human arginine-rich nuclear protein that colocalizes with spliceosome components. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8189–8193. doi: 10.1073/pnas.88.18.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993 Jan;12(1):97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Inagaki H., Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp Cell Res. 1994 Jun;212(2):426–430. doi: 10.1006/excr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Stournaras C., Blobel G. Heterotypic and homotypic associations between the nuclear lamins: site-specificity and control by phosphorylation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4325–4329. doi: 10.1073/pnas.85.12.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Guan R. J., Moss S. F., Arber N., Krajewski S., Reed J. C., Holt P. R. 30 KDa phosphorylated form of Bcl-2 protein in human colon. Oncogene. 1996 Jun 20;12(12):2605–2609. [PubMed] [Google Scholar]
- Guilly M. N., Bensussan A., Bourge J. F., Bornens M., Courvalin J. C. A human T lymphoblastic cell line lacks lamins A and C. EMBO J. 1987 Dec 1;6(12):3795–3799. doi: 10.1002/j.1460-2075.1987.tb02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilly M. N., Kolb J. P., Gosti F., Godeau F., Courvalin J. C. Lamins A and C are not expressed at early stages of human lymphocyte differentiation. Exp Cell Res. 1990 Jul;189(1):145–147. doi: 10.1016/0014-4827(90)90267-e. [DOI] [PubMed] [Google Scholar]
- Hirano T., Franzén B., Uryu K., Okuzawa K., Alaiya A. A., Vanky F., Rodrigues L., Ebihara Y., Kato H., Auer G. Detection of polypeptides associated with the histopathological differentiation of primary lung carcinoma. Br J Cancer. 1995 Oct;72(4):840–848. doi: 10.1038/bjc.1995.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hytiroglou P., Choi S. W., Theise N. D., Chaudhary N., Worman H. J., Thung S. N. The expression of nuclear lamins in human liver: an immunohistochemical study. Hum Pathol. 1993 Feb;24(2):169–172. doi: 10.1016/0046-8177(93)90296-s. [DOI] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Franke W. W. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol. 1988 Dec;47(2):283–290. [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kaufmann S. H., Mabry M., Jasti R., Shaper J. H. Differential expression of nuclear envelope lamins A and C in human lung cancer cell lines. Cancer Res. 1991 Jan 15;51(2):581–586. [PubMed] [Google Scholar]
- Krohne G., Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986 Jan;162(1):1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebel S., Lampron C., Royal A., Raymond Y. Lamins A and C appear during retinoic acid-induced differentiation of mouse embryonal carcinoma cells. J Cell Biol. 1987 Sep;105(3):1099–1104. doi: 10.1083/jcb.105.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Worman H. J. Expression of nuclear lamins in human tissues and cancer cell lines and transcription from the promoters of the lamin A/C and B1 genes. Exp Cell Res. 1997 Nov 1;236(2):378–384. doi: 10.1006/excr.1997.3735. [DOI] [PubMed] [Google Scholar]
- Lin F., Worman H. J. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995 May 20;27(2):230–236. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- Lin F., Worman H. J. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993 Aug 5;268(22):16321–16326. [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Paulin-Levasseur M., Giese G., Scherbarth A., Traub P. Expression of vimentin and nuclear lamins during the in vitro differentiation of human promyelocytic leukemia cells HL-60. Eur J Cell Biol. 1989 Dec;50(2):453–461. [PubMed] [Google Scholar]
- Paulin-Levasseur M., Scherbarth A., Giese G., Röser K., Bohn W., Traub P. Expression of nuclear lamins in mammalian somatic cells lacking cytoplasmic intermediate filament proteins. J Cell Sci. 1989 Mar;92(Pt 3):361–370. doi: 10.1242/jcs.92.3.361. [DOI] [PubMed] [Google Scholar]
- Paulin-Levasseur M., Scherbarth A., Traub U., Traub P. Lack of lamins A and C in mammalian hemopoietic cell lines devoid of intermediate filament proteins. Eur J Cell Biol. 1988 Oct;47(1):121–131. [PubMed] [Google Scholar]
- Pollard K. M., Chan E. K., Grant B. J., Sullivan K. F., Tan E. M., Glass C. A. In vitro posttranslational modification of lamin B cloned from a human T-cell line. Mol Cell Biol. 1990 May;10(5):2164–2175. doi: 10.1128/mcb.10.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röber R. A., Sauter H., Weber K., Osborn M. Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: distinction versus other somatic cells. J Cell Sci. 1990 Apr;95(Pt 4):587–598. doi: 10.1242/jcs.95.4.587. [DOI] [PubMed] [Google Scholar]
- Röber R. A., Weber K., Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989 Feb;105(2):365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Stewart C., Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987 Nov 6;51(3):383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Worman H. J., Lazaridis I., Georgatos S. D. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J Biol Chem. 1988 Aug 25;263(24):12135–12141. [PubMed] [Google Scholar]
- Wydner K. L., McNeil J. A., Lin F., Worman H. J., Lawrence J. B. Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization. Genomics. 1996 Mar 15;32(3):474–478. doi: 10.1006/geno.1996.0146. [DOI] [PubMed] [Google Scholar]
- Ye Q., Worman H. J. Protein-protein interactions between human nuclear lamins expressed in yeast. Exp Cell Res. 1995 Jul;219(1):292–298. doi: 10.1006/excr.1995.1230. [DOI] [PubMed] [Google Scholar]