Abstract
BACKGROUND—Keratinocyte growth factor (KGF) stimulates gastrointestinal epithelial cells in vivo, and is protective against gastrointestinal injury and colitis. Endogenous KGF is increased in inflammatory bowel disease (IBD), and may be an important mediator of mucosal repair. KGF is expressed by mesenchymal cells and activated intraepithelial lymphocytes (IEL). AIMS—To investigate the relative contributions of these cellular sources of KGF expression in IBD. METHODS—IELs and lamina propria lymphocytes (LPL) were isolated from inflamed and uninflamed IBD tissues. mRNA expression was determined by ribonuclease protection assay. In situ hybridisation was combined with immunohistochemistry to determine whether KGF mRNA was expressed by specific cell types in vivo. RESULTS—Low levels of KGF mRNA expression were detected in three of five IEL samples derived from inflamed tissue, but not in two IEL samples from uninflamed tissue. No KGF expression was detected in LPLs from either inflamed or uninflamed tissue. In contrast, KGF was expressed by primary cultures of human intestinal fibroblasts, and was induced by treatment with interleukin 1. CONCLUSIONS—The major source of KGF expression in IBD was lamina propria cells of non-immune origin, most likely fibroblasts and/or smooth muscle cells. Compared with these cell types, relatively little KGF synthesis was associated with IELs in inflamed IBD tissue. Keywords: fibroblasts; intestinal inflammation; intraepithelial lymphocytes; keratinocyte growth factor; lamina propria lymphocytes
Full Text
The Full Text of this article is available as a PDF (268.3 KB).
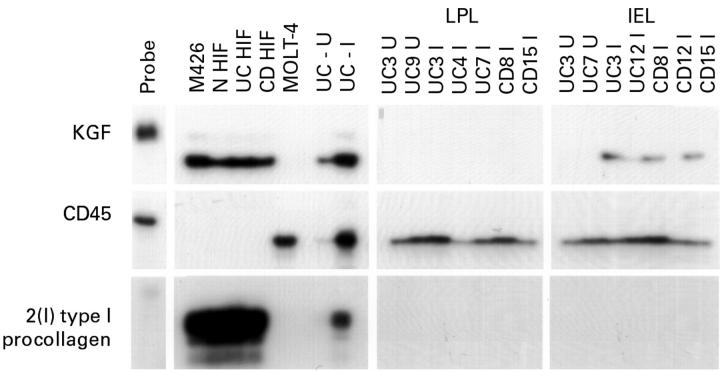
Figure 1 .
Analysis of keratinocyte growth factor (KGF), CD45, and α2(I) type I procollagen transcripts in lamina propria lymphocytes (LPL) and intraepithelial lymphocytes (IEL) isolated from uninvolved and inflamed inflammatory bowel disease tissues. RNAs (2 µg samples) were hybridised to 32P-labelled antisense probes and then digested with RNAse. Protected hybrids were resolved by electrophoresis through denaturing polyacrylamide gels. RNAs extracted from the embryonic lung fibroblast cell line, M426, and from three primary cultures of human intestinal fibroblasts isolated from normal (N), ulcerative colitis (UC), and Crohn's disease (CD) tissue, were included as positive controls for KGF and α2(I) type I procollagen expression. RNA isolated from the MOLT-4 T cell line was used as a positive control for CD45 expression. Total RNAs isolated from an uninvolved (U) and inflamed (I) region of ulcerative colitis were included for comparison. Exposure times were as follows: KGF, 72 h; CD45, 72 h; α2(I) type I procollagen, 24 h.
Figure 2 .
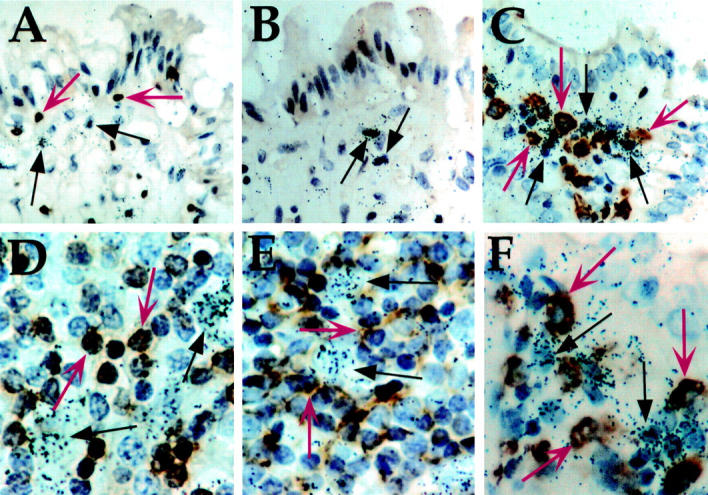
Joint localisation of keratinocyte growth factor (KGF) transcript expression and T cell, B cell, and macrophage immunohistochemical staining in uninvolved and inflamed inflammatory bowel disease tissues. Sections were processed for in situ hybridisation using an antisense KGF cRNA probe. The immunohistochemical label (brown) was visualised after incubation with antibodies directed against the CD3 (A and D), CD20 (B and E), or CD68 epitopes (C and F), which are specific for T cells, B cells, and macrophages, respectively. Following exposure to NTB-2 emulsion, slides were developed and counterstained with haematoxylin. Autoradiographic grain clusters show cells expressing KGF mRNA (arrows). (A-C) uninvolved tissue, original magnification × 156; (D-F) inflamed submucosa, original magnification × 187. Representative immunohistochemically labelled cells in each section are indicated by red arrows, and KGF expressing cells, identified by autoradiographic silver grain clusters, are indicated by black arrows.
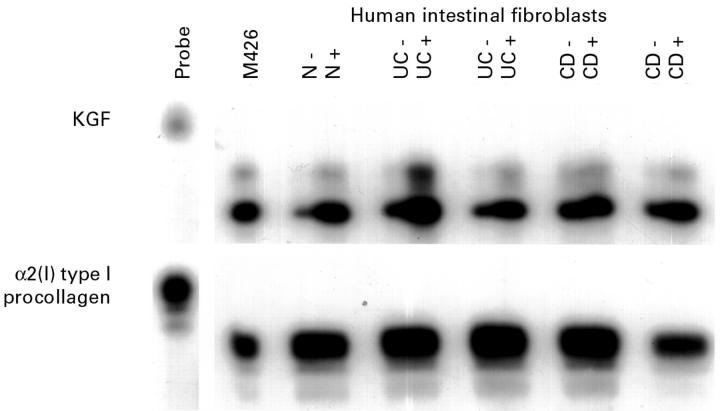
Figure 3 .
Induction of keratinocyte growth factor (KGF) mRNA by interleukin (IL) 1α in human intestinal fibroblasts (HIFs). Primary HIFs were derived from normal (N), and inflamed ulcerative colitis (UC) and Crohn's disease (CD) tissue. Cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum until 90% confluent, serum starved for 24 hours, and incubated with 20 ng/ml IL-1α for six hours (+) or left untreated (−). RNA was prepared and 10 µg samples were analysed by ribonuclease protection assay for KGF and α2(I) type I procollagen. Exposure times were 24 hours for the KGF autoradiograph, and six hours for α2(I) type I procollagen.
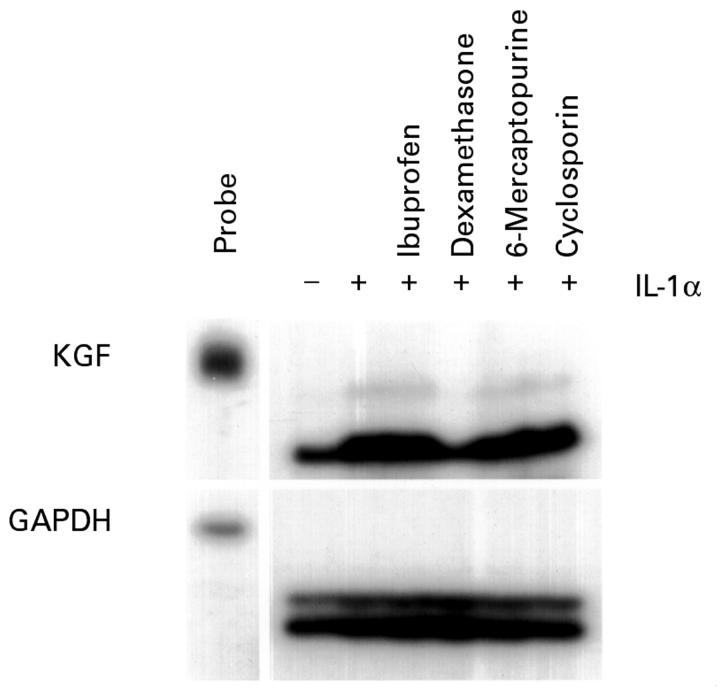
Figure 4 .
Induction of keratinocyte growth factor (KGF) RNA by interleukin (IL) 1α in 7-17 γδ dendritic epidermal T cells. Serum starved 7-17 cells were treated for 12 hours with IL-1α (20 ng/ml) in the presence or absence of ibuprofen (120 µM), dexamethasone (100 nM), 6-mercaptopurine (10 µM), or cyclosporin (1 µM). The control culture received vehicle alone. Total RNA was prepared and 10 µg samples were analysed by ribonuclease protection assay. Exposure times were 36 hours for the KGF autoradiograph, and six hours for glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
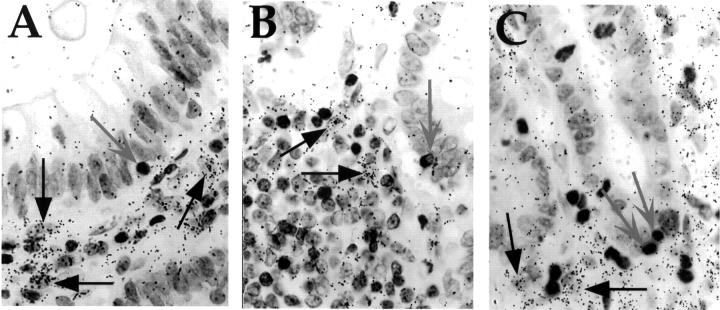
Figure 5 .
In situ hybridisation (ISH) analysis of keratinocyte growth factor (KGF) RNA expression in inflammatory bowel disease tissue of patients not receiving steroidal anti-inflammatory medication. Sections were processed for ISH using an antisense KGF cRNA probe, and then for immunohistochemistry using an anti-CD3 monoclonal antibody. Following exposure to NTB-2 emulsion, slides were developed and counterstained with haematoxylin. (A) Ulcerative colitis; (B) Crohn's disease; (C) ischaemic colitis. Intraepithelial lymphocytes are indicated by grey arrows, and representative KGF expressing cells by black arrows. Original magnification × 187.
Figure 6 .
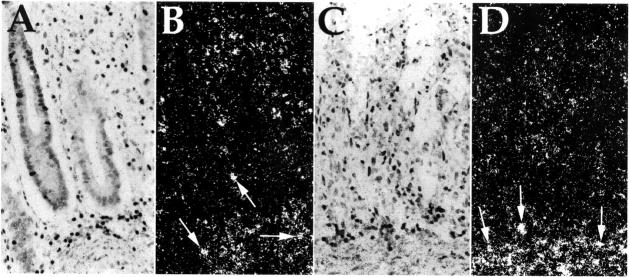
In situ hybridisation (ISH) analysis of keratinocyte growth factor (KGF) RNA expression in ischaemic colitis, shown in bright field (A and C) and corresponding dark field (B and D) micrographs of inflamed human intestinal tissue. Sections were processed for ISH using an antisense KGF cRNA probe. In (A), the mucosal epithelial layer is still intact, whereas in (C) the mucosal layer has been destroyed, and is absent. Numerous KGF expressing cells are present in the lamina propria of both tissue sections. Representative KGF expressing cells are indicated by arrows in (B) and (D). Stain: haematoxylin; original magnification × 62.5.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj-Elliott M., Breese E., Poulsom R., Fairclough P. D., MacDonald T. T. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn's disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol. 1997 Nov;151(5):1469–1476. [PMC free article] [PubMed] [Google Scholar]
- Bajaj-Elliott M., Poulsom R., Pender S. L., Wathen N. C., MacDonald T. T. Interactions between stromal cell--derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Invest. 1998 Oct 15;102(8):1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotnick S., Peoples G. E., Freeman M. R., Eberlein T. J., Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R., Feng L., Xia Y. Y., Chang J. C., Havran W. L. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996 Aug 1;157(3):985–992. [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Brauchle M., Angermeyer K., Hübner G., Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994 Nov;9(11):3199–3204. [PubMed] [Google Scholar]
- Brauchle M., Fässler R., Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol. 1995 Oct;105(4):579–584. doi: 10.1111/1523-1747.ep12323521. [DOI] [PubMed] [Google Scholar]
- Brauchle M., Madlener M., Wagner A. D., Angermeyer K., Lauer U., Hofschneider P. H., Gregor M., Werner S. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996 Aug;149(2):521–529. [PMC free article] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid M., Hoyle J. R., Csaky K. G., Rubin J. S. Glucocorticoids inhibit keratinocyte growth factor production in primary dermal fibroblasts. Endocrinology. 1996 Jun;137(6):2232–2237. doi: 10.1210/endo.137.6.8641170. [DOI] [PubMed] [Google Scholar]
- Chedid M., Rubin J. S., Csaky K. G., Aaronson S. A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994 Apr 8;269(14):10753–10757. [PubMed] [Google Scholar]
- Deusch K., Lüling F., Reich K., Classen M., Wagner H., Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991 Apr;21(4):1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- Farrell C. L., Bready J. V., Rex K. L., Chen J. N., DiPalma C. R., Whitcomb K. L., Yin S., Hill D. C., Wiemann B., Starnes C. O. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998 Mar 1;58(5):933–939. [PubMed] [Google Scholar]
- Finch P. W., Cunha G. R., Rubin J. S., Wong J., Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995 Jun;203(2):223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Murphy F., Cardinale I., Krueger J. G. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am J Pathol. 1997 Dec;151(6):1619–1628. [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Pricolo V., Wu A., Finkelstein S. D. Increased expression of keratinocyte growth factor messenger RNA associated with inflammatory bowel disease. Gastroenterology. 1996 Feb;110(2):441–451. doi: 10.1053/gast.1996.v110.pm8566591. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., Kawabata S., Hiroi T., Yamamoto M., McGhee J. R., Nishikawa S., Kiyono H. Interleukin 2 (IL-2) and interleukin 7 (IL-7) reciprocally induce IL-7 and IL-2 receptors on gamma delta T-cell receptor-positive intraepithelial lymphocytes. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3613–3618. doi: 10.1073/pnas.93.8.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Masuda T., Ohtani H., Sasaki I., Funayama Y., Matsuno S., Nagura H. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 1991 Sep;101(3):670–678. doi: 10.1016/0016-5085(91)90524-o. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Steinle A., Bauer S., Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998 Mar 13;279(5357):1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989 Dec;30(6):665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. Current concepts in mucosal immunity. III. Ontogeny and function of gamma delta T cells in the intestine. Am J Physiol. 1998 Mar;274(3 Pt 1):G455–G458. doi: 10.1152/ajpgi.1998.274.3.G455. [DOI] [PubMed] [Google Scholar]
- Komano H., Fujiura Y., Kawaguchi M., Matsumoto S., Hashimoto Y., Obana S., Mombaerts P., Tonegawa S., Yamamoto H., Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel W. A., Takashima A., Bonyhadi M., Bergstresser P. R., Allison J. P., Tigelaar R. E., Tucker P. W. Regulation of T-cell receptor gamma-chain RNA expression in murine Thy-1+ dendritic epidermal cells. Nature. 1987 Jul 16;328(6127):263–266. doi: 10.1038/328263a0. [DOI] [PubMed] [Google Scholar]
- McVay L. D., Li B., Biancaniello R., Creighton M. A., Bachwich D., Lichtenstein G., Rombeau J. L., Carding S. R. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med. 1997 Mar;3(3):183–203. [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Puddington L., Olson S., Lefrançois L. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity. 1994 Dec;1(9):733–739. doi: 10.1016/s1074-7613(94)80015-4. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust C., Peña S., Kluin P., Koning F. Gamma delta T cells in coeliac disease. Res Immunol. 1990 Sep;141(7):668–671. doi: 10.1016/0923-2494(90)90079-e. [DOI] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., Diss T. C., MacDonald T. T. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor gamma/delta heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989 Jul;19(7):1335–1338. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., MacDonald T. T., Thomas A. J., Walker-Smith J. A. Gamma/delta T cells and the diagnosis of coeliac disease. Clin Exp Immunol. 1991 Jul;85(1):109–113. doi: 10.1111/j.1365-2249.1991.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong S. A., Pizarro T. T., Klein J. S., Cominelli F., Fiocchi C. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology. 1998 Jun;114(6):1244–1256. doi: 10.1016/s0016-5085(98)70431-7. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Calabrese A., Smart C. J., Oakes D. J., Howdle P. D., Crabtree J. E., Losowsky M. S., Lancaster F., Boylston A. W. Gamma delta T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Heliobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin Exp Immunol. 1991 Jun;84(3):440–444. [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Iwao Y., Tsuchiya M., Ishikawa H., Aiso S., Hibi T., Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995 Jun;95(6):2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Alberts G. F., Chedid M., Taylor W. G., DeMartino S., Rubin J. S. Differential expression of the keratinocyte growth factor (KGF) and KGF receptor genes in human vascular smooth muscle cells and arteries. J Cell Physiol. 1997 Dec;173(3):380–386. doi: 10.1002/(SICI)1097-4652(199712)173:3<380::AID-JCP10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Amano M., McGhee J. R., Beagley K. W., Kiyono H. Cytokine synthesis and apoptosis by intestinal intraepithelial lymphocytes: signaling of high density alpha beta T cell receptor+ and gamma delta T cell receptor+ T cells via T cell receptor-CD3 complex results in interferon-gamma and interleukin-5 production, while low density T cells undergo DNA fragmentation. Eur J Immunol. 1994 Jun;24(6):1301–1306. doi: 10.1002/eji.1830240609. [DOI] [PubMed] [Google Scholar]
- Zeeh J. M., Procaccino F., Hoffmann P., Aukerman S. L., McRoberts J. A., Soltani S., Pierce G. F., Lakshmanan J., Lacey D., Eysselein V. E. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996 Apr;110(4):1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]
- Zhao X. M., Yeoh T. K., Hiebert M., Frist W. H., Miller G. G. The expression of acidic fibroblast growth factor (heparin-binding growth factor-1) and cytokine genes in human cardiac allografts and T cells. Transplantation. 1993 Nov;56(5):1177–1182. doi: 10.1097/00007890-199311000-00025. [DOI] [PubMed] [Google Scholar]