Abstract
BACKGROUND—Sulindac regresses colorectal adenomas in patients with familial adenomatous polyposis (FAP), although the mechanism of polyp regression is unclear. AIMS—To determine whether differences occur in alteration of rectal epithelial apoptotic index and expression of apoptosis related proteins in FAP patients treated with sulindac compared with placebo. PATIENTS—Twenty one FAP patients; 12 had not undergone colectomy. METHODS—Patients with FAP were treated with sulindac 150 mg orally twice a day for three months (n=10) or placebo (n=11). Colorectal polyp number was determined and biopsies of the normal rectal mucosa were performed before and after three months of treatment. Response to treatment and alteration of the apoptotic ratio (index in base of crypt divided by index in surface epithelium) were evaluated. Bcl-2, bax, p21/WAF-1, and p53 proteins were assessed semiquantitatively by immunohistochemistry. RESULTS—Significant decreases in polyp number and in the apoptotic ratio were seen in patients treated with sulindac compared with controls. The mean percentage change in polyp number from baseline was −46% in the sulindac group and +13% in the placebo group (p=0.005). Mean percentage change in the apoptotic ratio was −8% and +25% in the sulindac and placebo treated patients, respectively (p=0.004). No differences in expression or compartmentalisation of apoptosis related proteins were noted between treatment groups. CONCLUSIONS—Sulindac regression of colorectal adenomas is accompanied by alteration of the rectal epithelial apoptotic ratio with relative increase in apoptosis in surface cells compared with the deeper crypt. The utility of the apoptotic ratio as an intermediate biomarker for colorectal tumorigenesis deserves further study. Keywords: apoptosis; familial adenomatous polyposis; sulindac; intermediate biomarker; tumorigenesis
Full Text
The Full Text of this article is available as a PDF (156.0 KB).
Figure 1 .

(A) Apoptotic cell with chromatin condensation, separation of the cell from adjacent enterocytes, formation of apoptotic body, cytoplasmic swelling. (B) WAF-1/p21 expression. (C) bcl-2 expression. (D) bax expression.
Figure 2 .

Decrease in apoptotic ratio with relatively greater apoptosis at the luminal surface compared with the base of the crypt seen in patients treated with sulindac compared with controls. Biopsy specimens were taken from the flat, normal appearing colorectal mucosa. Arrow indicates the patient who developed rectal cancer while on treatment with sulindac.
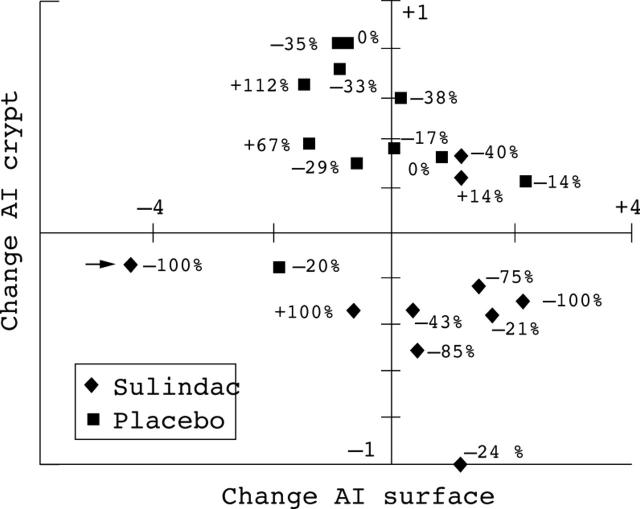
Figure 3 .
Change in apoptotic index at surface (x axis) plotted against change in apoptotic index at crypt (y axis) per patient treated with sulindac or placebo. Each observation is labelled with the change in polyp number. Arrow indicates the patient who developed rectal cancer while on treatment with sulindac.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Han E. K., Sgambato A., Piazza G. A., Delohery T. M., Begemann M., Weghorst C. M., Kim N. H., Pamukcu R., Ahnen D. J. A K-ras oncogene increases resistance to sulindac-induced apoptosis in rat enterocytes. Gastroenterology. 1997 Dec;113(6):1892–1900. doi: 10.1016/s0016-5085(97)70008-8. [DOI] [PubMed] [Google Scholar]
- Baas I. O., Mulder J. W., Offerhaus G. J., Vogelstein B., Hamilton S. R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994 Jan;172(1):5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- Baretton G. B., Diebold J., Christoforis G., Vogt M., Müller C., Dopfer K., Schneiderbanger K., Schmidt M., Löhrs U. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Aspects of carcinogenesis and prognostic significance. Cancer. 1996 Jan 15;77(2):255–264. doi: 10.1002/(SICI)1097-0142(19960115)77:2<255::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bayer B. M., Beaven M. A. Evidence that indomethacin reversibly inhibits cell growth in the G1 phase of the cell cycle. Biochem Pharmacol. 1979;28(3):441–443. doi: 10.1016/0006-2952(79)90112-6. [DOI] [PubMed] [Google Scholar]
- Bayer B. M., Kruth H. S., Vaughan M., Beaven M. A. Arrest of cultured cells in the G1 phase of the cell cycle by indomethacin. J Pharmacol Exp Ther. 1979 Jul;210(1):106–111. [PubMed] [Google Scholar]
- Bedi A., Pasricha P. J., Akhtar A. J., Barber J. P., Bedi G. C., Giardiello F. M., Zehnbauer B. A., Hamilton S. R., Jones R. J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995 May 1;55(9):1811–1816. [PubMed] [Google Scholar]
- Bullions L. C., Levine A. J. The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol. 1998 Jan;10(1):81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Chan T. A., Morin P. J., Vogelstein B., Kinzler K. W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. A., Morin P. J., Vogelstein B., Kinzler K. W. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Offerhaus G. J., DuBois R. N. The role of nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Offerhaus J. A., Tersmette A. C., Hylind L. M., Krush A. J., Brensinger J. D., Booker S. V., Hamilton S. R. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996 Apr;38(4):578–581. doi: 10.1136/gut.38.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello F. M., Spannhake E. W., DuBois R. N., Hylind L. M., Robinson C. R., Hubbard W. C., Hamilton S. R., Yang V. W. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1998 Feb;43(2):311–316. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Stampfer M. J., Colditz G. A., Ascherio A., Willett W. C. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994 Aug 15;121(4):241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- Goldberg Y., Nassif I. I., Pittas A., Tsai L. L., Dynlacht B. D., Rigas B., Shiff S. J. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996 Feb 15;12(4):893–901. [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994 Dec;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Hanif R., Pittas A., Feng Y., Koutsos M. I., Qiao L., Staiano-Coico L., Shiff S. I., Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996 Jul 26;52(2):237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Wong M. H., Gordon J. I. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996 Apr 15;10(8):985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- Hial V., De Mello M. C., Horakova Z., Beaven M. A. Antiproliferative activity of anti-inflammatory drugs in two mammalian cell culture lines. J Pharmacol Exp Ther. 1977 Aug;202(2):446–454. [PubMed] [Google Scholar]
- Jacoby R. F., Marshall D. J., Newton M. A., Novakovic K., Tutsch K., Cole C. E., Lubet R. A., Kelloff G. J., Verma A., Moser A. R. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996 Feb 15;56(4):710–714. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997 Mar 21;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Miyashita T., Wang H. G., Reed J. C. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994 Dec;145(6):1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Labayle D., Fischer D., Vielh P., Drouhin F., Pariente A., Bories C., Duhamel O., Trousset M., Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991 Sep;101(3):635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer. 1974 Sep;34(3):suppl–suppl:888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mahmoud N. N., Boolbol S. K., Bilinski R. T., Martucci C., Chadburn A., Bertagnolli M. M. Apc gene mutation is associated with a dominant-negative effect upon intestinal cell migration. Cancer Res. 1997 Nov 15;57(22):5045–5050. [PubMed] [Google Scholar]
- Mills S. J., Shepherd N. A., Hall P. A., Hastings A., Mathers J. C., Gunn A. Proliferative compartment deregulation in the non-neoplastic colonic epithelium of familial adenomatous polyposis. Gut. 1995 Mar;36(3):391–394. doi: 10.1136/gut.36.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorghen M., Ince P., Finney K. J., Sunter J. P., Appleton D. R., Watson A. J. A protective effect of sulindac against chemically-induced primary colonic tumours in mice. J Pathol. 1988 Dec;156(4):341–347. doi: 10.1002/path.1711560411. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Vogelstein B., Kinzler K. W. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier J. F., Perret A. G., Vindimian M., Dumollard J. M., Balique J. G., Perpoint B., Boucheron S. An immunohistochemical study of the simultaneous expression of bcl-2 and p53 oncoproteins in epithelial tumors of the colon and rectum. Arch Pathol Lab Med. 1996 Jul;120(7):654–659. [PubMed] [Google Scholar]
- Moss S. F., Liu T. C., Petrotos A., Hsu T. M., Gold L. I., Holt P. R. Inward growth of colonic adenomatous polyps. Gastroenterology. 1996 Dec;111(6):1425–1432. doi: 10.1016/s0016-5085(96)70003-3. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Scholes J. V., Holt P. R. Abnormalities of epithelial apoptosis in multistep colorectal neoplasia demonstrated by terminal deoxyuridine nick end labeling. Dig Dis Sci. 1996 Nov;41(11):2238–2247. doi: 10.1007/BF02071407. [DOI] [PubMed] [Google Scholar]
- Nugent K. P., Farmer K. C., Spigelman A. D., Williams C. B., Phillips R. K. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993 Dec;80(12):1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- Pasricha P. J., Bedi A., O'Connor K., Rashid A., Akhtar A. J., Zahurak M. L., Piantadosi S., Hamilton S. R., Giardiello F. M. The effects of sulindac on colorectal proliferation and apoptosis in familial adenomatous polyposis. Gastroenterology. 1995 Sep;109(3):994–998. doi: 10.1016/0016-5085(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Piazza G. A., Rahm A. K., Finn T. S., Fryer B. H., Li H., Stoumen A. L., Pamukcu R., Ahnen D. J. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997 Jun 15;57(12):2452–2459. [PubMed] [Google Scholar]
- Piazza G. A., Rahm A. L., Krutzsch M., Sperl G., Paranka N. S., Gross P. H., Brendel K., Burt R. W., Alberts D. S., Pamukcu R. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995 Jul 15;55(14):3110–3116. [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Indomethacin treatment of rats with dimethylhydrazine-induced intestinal tumors. Cancer Treat Rep. 1980;64(12):1323–1327. [PubMed] [Google Scholar]
- Polyak K., Hamilton S. R., Vogelstein B., Kinzler K. W. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol. 1996 Aug;149(2):381–387. [PMC free article] [PubMed] [Google Scholar]
- Rao C. V., Rivenson A., Simi B., Zang E., Kelloff G., Steele V., Reddy B. S. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995 Apr 1;55(7):1464–1472. [PubMed] [Google Scholar]
- Reddy B. S., Rao C. V., Rivenson A., Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993 Aug;14(8):1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- Rigau J., Piqué J. M., Rubio E., Planas R., Tarrech J. M., Bordas J. M. Effects of long-term sulindac therapy on colonic polyposis. Ann Intern Med. 1991 Dec 15;115(12):952–954. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- Samaha H. S., Kelloff G. J., Steele V., Rao C. V., Reddy B. S. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997 Apr 1;57(7):1301–1305. [PubMed] [Google Scholar]
- Shiff S. J., Qiao L., Tsai L. L., Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995 Jul;96(1):491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope F. A., Roddey G., McDonnell T. J., Shen Y., Cleary K. R., Stephens L. C. Increased apoptosis accompanies neoplastic development in the human colorectum. Clin Cancer Res. 1996 Dec;2(12):1999–2006. [PubMed] [Google Scholar]
- Sinicrope F. A., Ruan S. B., Cleary K. R., Stephens L. C., Lee J. J., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995 Jan 15;55(2):237–241. [PubMed] [Google Scholar]
- Slebos R. J., Baas I. O., Clement M., Polak M., Mulder J. W., van den Berg F. M., Hamilton S. R., Offerhaus G. J. Clinical and pathological associations with p53 tumour-suppressor gene mutations and expression of p21WAF1/Cip1 in colorectal carcinoma. Br J Cancer. 1996 Jul;74(2):165–171. doi: 10.1038/bjc.1996.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnesi M. T., Tonelli F., Dolara P., Caderni G., Valanzano R., Anastasi A., Bianchini F. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology. 1994 Feb;106(2):362–366. doi: 10.1016/0016-5085(94)90593-2. [DOI] [PubMed] [Google Scholar]
- Sparks A. B., Morin P. J., Vogelstein B., Kinzler K. W. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998 Mar 15;58(6):1130–1134. [PubMed] [Google Scholar]
- Thun M. J., Namboodiri M. M., Heath C. W., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991 Dec 5;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Loughry R. W. Sulindac for polyposis of the colon. J Surg Oncol. 1983 Sep;24(1):83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- Winde G., Schmid K. W., Brandt B., Müller O., Osswald H. Clinical and genomic influence of sulindac on rectal mucosa in familial adenomatous polyposis. Dis Colon Rectum. 1997 Oct;40(10):1156–1169. doi: 10.1007/BF02055161. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- van de Schepop H. A., de Jong J. S., van Diest P. J., Baak J. P. Counting of apoptotic cells: a methodological study in invasive breast cancer. Clin Mol Pathol. 1996 Aug;49(4):M214–M217. doi: 10.1136/mp.49.4.m214. [DOI] [PMC free article] [PubMed] [Google Scholar]