Abstract
BACKGROUND—In many transplant centres lamivudine is an important component of prophylaxis against, and treatment of, hepatitis B virus (HBV) graft infection. Drug resistant HBV species with specific polymerase mutations may emerge during lamivudine treatment. AIMS—To examine the clinical consequences of graft infection by lamivudine resistant virus. METHODS—The clinical course of four liver transplant patients who developed graft infection with lamivudine resistant virus was reviewed. The response of HBV infection to reduction of immunosuppression and to manipulation of antiviral therapy was assessed. For each patient, serum viral titre was measured and the viral polymerase gene was sequenced at multiple time points. RESULTS—High serum titres were observed following emergence of the lamivudine resistant species. Wild type HBV re-emerged as the dominant serum species after lamivudine withdrawal. All patients developed liver failure, and onset of liver dysfunction was observed when resistant virus was the dominant serum species. In three patients, liver recovery was observed when immunosuppression was stopped and when alternative antivirals were given. Wild type virus appeared to respond to ganciclovir, and to reintroduction of lamivudine. For one patient, introduction of famciclovir was associated with clinical, virological, and histological response. CONCLUSIONS—Failure of lamivudine prophylaxis may identify patients at special risk for the development of severe graft infection. Treatment of graft reinfection should include reduction of immunosuppression, and systematic exposure to alternative antivirals. Viral quantitation and genetic sequencing are essential components of therapeutic monitoring. Keywords: hepatitis B virus; lamivudine; prophylaxis; reinfection
Full Text
The Full Text of this article is available as a PDF (140.4 KB).
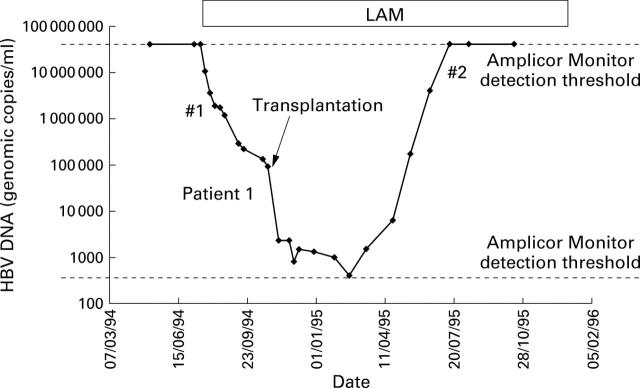
Figure 1 .
Response of patient 1 to treatment with lamivudine (LAM). Genetic sequencing was undertaken at time points #1 and #2.
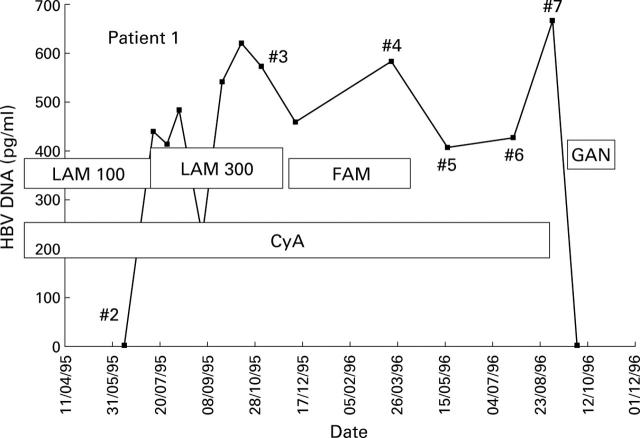
Figure 2 .
Response observed in patient 1 (HBV titre measured with Abbott Genostics assay) to administration of increased lamivudine (LAM) dose, famciclovir (FAM), ganciclovir (GAN), and cessation of immunosuppression. HBV was sequenced at multiple time points #2 to #7. CyA, cyclosporin.
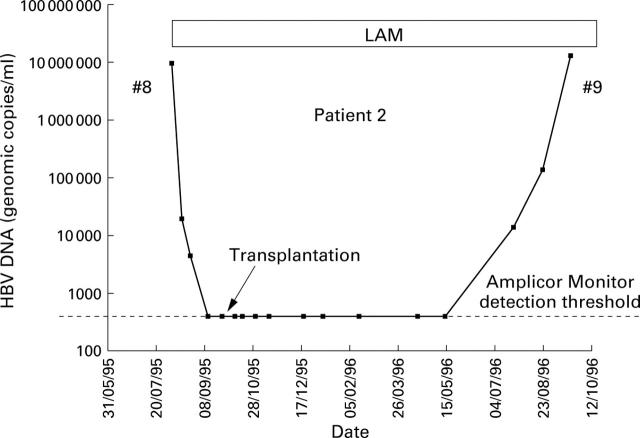
Figure 3 .
Response of patient 2 to treatment with lamivudine (LAM).
Figure 4 .
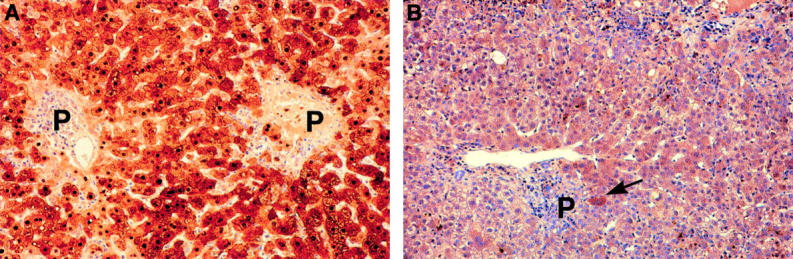
Histological response of patient 3 to famciclovir. Immunoperoxide staining for hepatitis B core antigen (HBcAg). (A) Biopsy performed three days before treatment with famciclovir shows panacinar staining for HBcAg (nuclear and cytoplasmic). (B) Histology 20 days after starting treatment. Note the remarkable reduction of staining for HBcAg. Occasional hepatocytes still show positive cytoplasmic staining (arrow). P, portal tract.
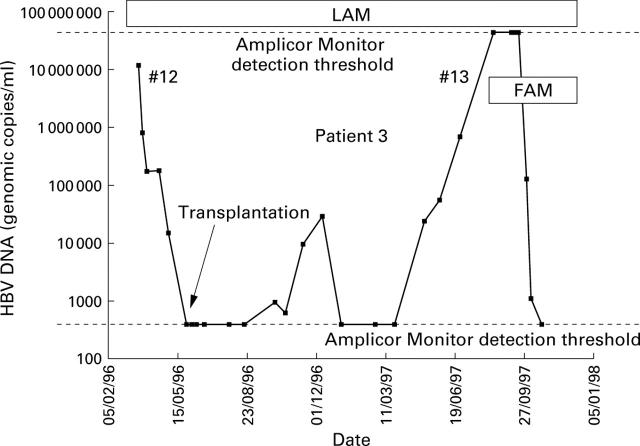
Figure 5 .
Response of patient 3 to lamivudine (LAM) treatment. #13 represents emergence of resistant species. Treatment with lamivudine was sustained and famciclovir (FAM) was added.
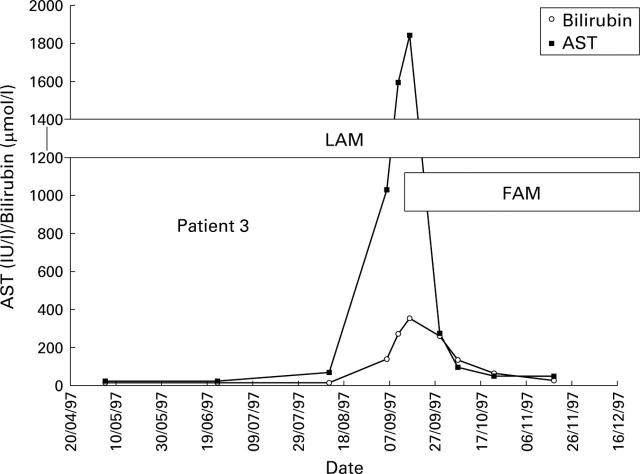
Figure 6 .
Biochemical response observed for patient 3 who developed liver failure with jaundice and ascites following emergence of lamivudine (LAM) resistant virus. Addition of famciclovir (FAM) was associated with dramatic biochemical improvement. AST, aspartate aminotransferase.
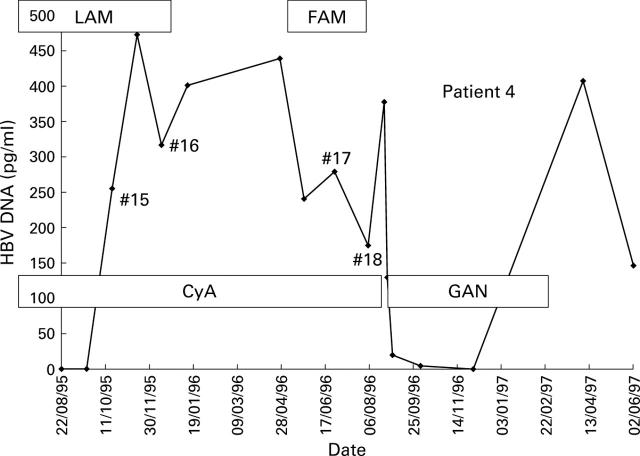
Figure 7 .
Response of patient 4 following emergence of lamivudine (LAM) resistant HBV (specimen #15) to treatment with famciclovir (FAM) and ganciclovir (GAN). CyA, cyclosporin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye T. T., Bartholomeusz A., Shaw T., Bowden S., Breschkin A., McMillan J., Angus P., Locarnini S. Hepatitis B virus polymerase mutations during antiviral therapy in a patient following liver transplantation. J Hepatol. 1997 May;26(5):1148–1153. doi: 10.1016/s0168-8278(97)80125-0. [DOI] [PubMed] [Google Scholar]
- Bartholomew M. M., Jansen R. W., Jeffers L. J., Reddy K. R., Johnson L. C., Bunzendahl H., Condreay L. D., Tzakis A. G., Schiff E. R., Brown N. A. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997 Jan 4;349(9044):20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- Benhamou Y., Dohin E., Lunel-Fabiani F., Poynard T., Huraux J. M., Katlama C., Opolon P., Gentilini M. Efficacy of lamivudine on replication of hepatitis B virus in HIV-infected patients. Lancet. 1995 Feb 11;345(8946):396–397. doi: 10.1016/s0140-6736(95)90388-7. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Perrillo R. P., Schiff E. R., Bartholomew M., Vicary C., Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995 Dec 21;333(25):1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- Gish R. G., Lau J. Y., Brooks L., Fang J. W., Steady S. L., Imperial J. C., Garcia-Kennedy R., Esquivel C. O., Keeffe E. B. Ganciclovir treatment of hepatitis B virus infection in liver transplant recipients. Hepatology. 1996 Jan;23(1):1–7. doi: 10.1002/hep.510230101. [DOI] [PubMed] [Google Scholar]
- Grellier L., Mutimer D., Ahmed M., Brown D., Burroughs A. K., Rolles K., McMaster P., Beranek P., Kennedy F., Kibbler H. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996 Nov 2;348(9036):1212–1215. doi: 10.1016/s0140-6736(96)04444-3. [DOI] [PubMed] [Google Scholar]
- Honkoop P., Niesters H. G., de Man R. A., Osterhaus A. D., Schalm S. W. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997 Jun;26(6):1393–1395. doi: 10.1016/s0168-8278(97)80476-x. [DOI] [PubMed] [Google Scholar]
- Krüger M., Tillmann H. L., Trautwein C., Bode U., Oldhafer K., Maschek H., Böker K. H., Broelsch C. E., Pichlmayr R., Manns M. P. Famciclovir treatment of hepatitis B virus recurrence after liver transplantation: a pilot study. Liver Transpl Surg. 1996 Jul;2(4):253–262. doi: 10.1002/lt.500020402. [DOI] [PubMed] [Google Scholar]
- Lai C. L., Ching C. K., Tung A. K., Li E., Young J., Hill A., Wong B. C., Dent J., Wu P. C. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen carriers: a placebo-controlled trial. Hepatology. 1997 Jan;25(1):241–244. doi: 10.1002/hep.510250144. [DOI] [PubMed] [Google Scholar]
- Ling R., Mutimer D., Ahmed M., Boxall E. H., Elias E., Dusheiko G. M., Harrison T. J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996 Sep;24(3):711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- McCaughan G., Angus P., Bowden S., Shaw T., Breschkin A., Sheil R., Locarnini S. Retransplantation for precore mutant-related chronic hepatitis B infection: prolonged survival in a patient receiving sequential ganciclovir/famciclovir therapy. Liver Transpl Surg. 1996 Nov;2(6):472–474. doi: 10.1002/lt.500020611. [DOI] [PubMed] [Google Scholar]
- Mutimer D., Pillay D., Dragon E., Tang H., Ahmed M., O'Donnell K., Shaw J., Burroughs N., Rand D., Cane P. High pre-treatment serum hepatitis B virus titre predicts failure of lamivudine prophylaxis and graft re-infection after liver transplantation. J Hepatol. 1999 Apr;30(4):715–721. doi: 10.1016/s0168-8278(99)80204-9. [DOI] [PubMed] [Google Scholar]
- Tipples G. A., Ma M. M., Fischer K. P., Bain V. G., Kneteman N. M., Tyrrell D. L. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996 Sep;24(3):714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]