Abstract
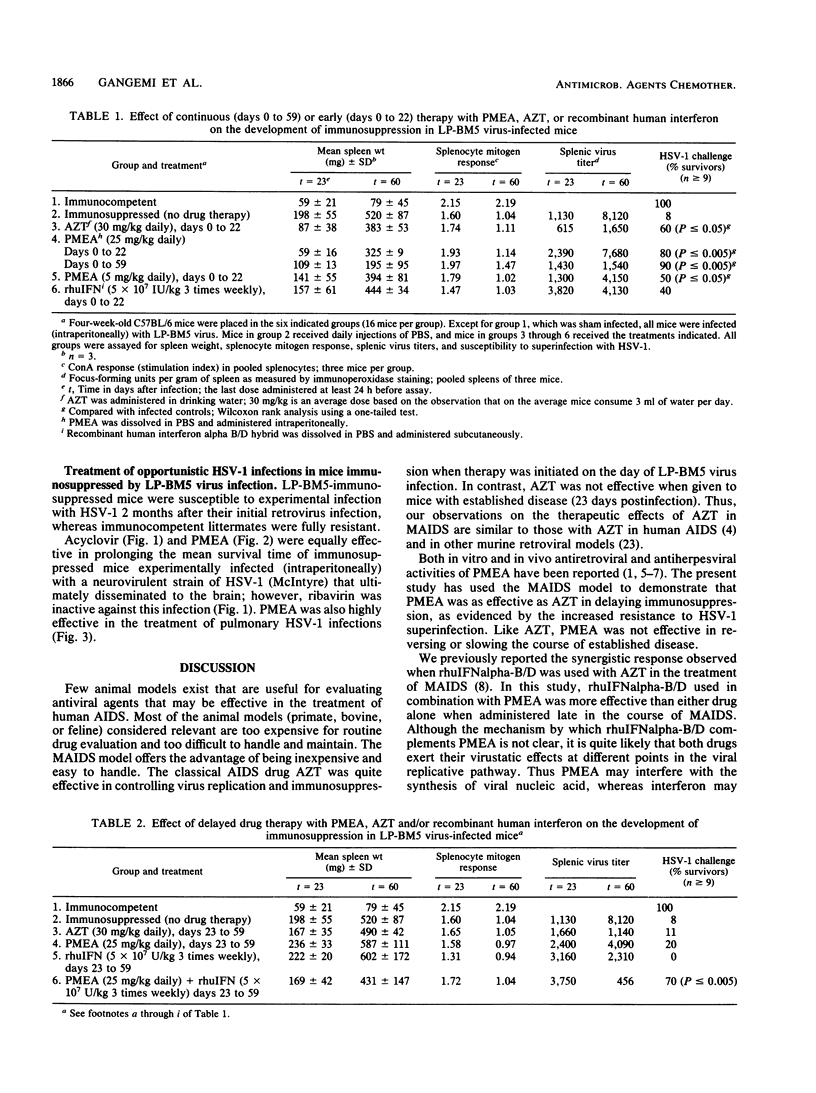
The murine model of acquired immunodeficiency disease was used to evaluate both the antiretroviral and antiherpetic activities of the acyclic nucleotide analog 9-(2-phosphonylmethoxyethyl)adenine (PMEA). The antiretroviral activity of PMEA was compared with that of azidothymidine (AZT) in mice receiving the drug either immediately after infection or at late times in disease progression. Both AZT (oral, 30 mg/kg) and PMEA (parenteral, 25 and 5 mg/kg) were effective in slowing the development of disease when administered daily beginning on the day of infection. In contrast, neither drug alone was effective in modifying disease outcome when administered several weeks after viral infection. Human recombinant alpha interferon (rhuIFN alpha-B/D at 5 x 10(7) U/kg) was also ineffective when administered late in the course of disease. However, when administered in combination, both alpha interferon and PMEA (25 mg/kg) were able to suppress disease progression even when treatment was initiated as late as 3 weeks postinfection. Mice that were immunocompromised due to LP-BM5 virus infection were highly susceptible to acute (lethal) infection with herpes simplex virus type 1, whereas their immunocompetent littermates were not. PMEA was as effective as acyclovir in the treatment of opportunistic herpes simplex virus type 1 infections in LP-BM5 virus-infected mice. Thus, like AZT, PMEA was effective against retrovirus infection, and, like acyclovir, PMEA was effective against herpes simplex virus type 1 infection. This gives PMEA the unique potential of being useful in the treatment of opportunistic herpes simplex virus infections as well as the underlying retroviral disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller R. M., Yetter R. A., Fredrickson T. N., Morse H. C., 3rd Abrogation of resistance to severe mousepox in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. J Virol. 1987 Feb;61(2):383–387. doi: 10.1128/jvi.61.2.383-387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988 Oct;62(10):3779–3788. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh-Kirk T., Doi P., Andrews E., Nusinoff-Lehrman S., Tilson H., Hoth D., Barry D. W. Survival experience among patients with AIDS receiving zidovudine. Follow-up of patients in a compassionate plea program. JAMA. 1988 Nov 25;260(20):3009–3015. [PubMed] [Google Scholar]
- De Clercq E., Holý A., Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob Agents Chemother. 1989 Feb;33(2):185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P. C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986 Oct 2;323(6087):464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Sakuma T., Baba M., Pauwels R., Balzarini J., Rosenberg I., Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987 Dec;8(5-6):261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- Gangemi J. D., Nachtigal M., Barnhart D., Krech L., Jani P. Therapeutic efficacy of liposome-encapsulated ribavirin and muramyl tripeptide in experimental infection with influenza or herpes simplex virus. J Infect Dis. 1987 Mar;155(3):510–517. doi: 10.1093/infdis/155.3.510. [DOI] [PubMed] [Google Scholar]
- Haas M., Meshorer A. Reticulum cell neoplasms induced in C57BL/6 mice by cultured virus grown in stromal hematopoietic cell lines. J Natl Cancer Inst. 1979 Aug;63(2):427–439. [PubMed] [Google Scholar]
- Haas M., Reshef T. Non-thymic malignant lymphomas induced in C57BL/6 mice by cloned dualtropic viruses isolated from hematopoietic stromal cell lines. Eur J Cancer. 1980 Jul;16(7):909–917. doi: 10.1016/0014-2964(80)90329-1. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinken S. P., Fredrickson T. N., Hartley J. W., Yetter R. A., Morse H. C., 3rd Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988 Feb 15;140(4):1123–1131. [PubMed] [Google Scholar]
- Meister A., Uzé G., Mogensen K. E., Gresser I., Tovey M. G., Grütter M., Meyer F. Biological activities and receptor binding of two human recombinant interferons and their hybrids. J Gen Virol. 1986 Aug;67(Pt 8):1633–1643. doi: 10.1099/0022-1317-67-8-1633. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Animal models for retrovirus-induced immunodeficiency disease. Immunol Invest. 1986 May;15(3):233–261. doi: 10.3109/08820138609026687. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nachtigal M., Caulfield J. B. Early and late pathologic changes in the adrenal glands of mice after infection with herpes simplex virus type 1. Am J Pathol. 1984 May;115(2):175–185. [PMC free article] [PubMed] [Google Scholar]
- Nexo B. A. A plaque assay for murine leukemia virus using enzyme-coupled antibodies. Virology. 1977 Apr;77(2):849–852. doi: 10.1016/0042-6822(77)90504-9. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Twomey P., Hill S., Jonasson J., Beardsley T., Haas M. Immunopathology of B-cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV). Am J Pathol. 1982 Jun;107(3):362–377. [PMC free article] [PubMed] [Google Scholar]
- Ruprecht R. M., O'Brien L. G., Rossoni L. D., Nusinoff-Lehrman S. Suppression of mouse viraemia and retroviral disease by 3'-azido-3'-deoxythymidine. Nature. 1986 Oct 2;323(6087):467–469. doi: 10.1038/323467a0. [DOI] [PubMed] [Google Scholar]


