Abstract
BACKGROUND—Butyrate oxidation within the colonocyte is selectively inhibited by hydrogen sulphide, reproducing the metabolic lesion observed in active ulcerative colitis. AIMS—To study generation of hydrogen sulphide by sulphate reducing bacteria (SRB) and the effects of 5-aminosalicylic acid (5-ASA) in patients with ulcerative colitis in order to identify a role of this noxious agent in pathogenesis. PATIENTS—Fresh faeces were obtained from 37 patients with ulcerative colitis (23 with active disease) and 16 healthy controls. METHODS—SRB were enumerated from fresh faecal slurries and measurements made of sulphate reducing activity, and sulphate and hydrogen sulphide concentrations. The effect of 5-ASA on hydrogen sulphide production was studied in vitro. RESULTS—All controls and patients with active ulcerative colitis carried SRB and total viable counts were significantly related to the clinical severity grade. SRB were of two distinct types: rapidly growing strains (desulfovibrios) which showed high sulphate reduction rates, present in 30% of patients with ulcerative colitis and 44% of controls; and slow growing strains which had little activity. In vitro, 5-ASA inhibited sulphide production in a dose dependent manner; in patients with ulcerative colitis not on these drugs faecal sulphide was significantly higher than in controls (0.55 versus 0.25 mM, p=0.027). CONCLUSIONS—Counts and carriage rates of SRB in faeces of patients with ulcerative colitis are not significantly different from those in controls. SRB metabolism is not uniform between strains and alternative sources of hydrogen sulphide production exist in the colonic lumen which may be similarly inhibited by 5-ASA. The evidence for hydrogen sulphide as a metabolic toxin in ulcerative colitis remains circumstantial. Keywords: colitis; sulphate; sulphide; bacteria; fermentation; salicylate
Full Text
The Full Text of this article is available as a PDF (128.4 KB).
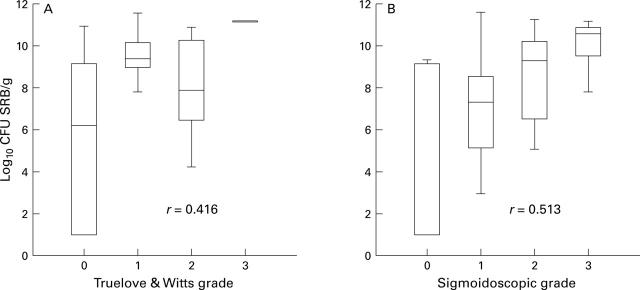
Figure 1 .
Box and whisker plots of total viable counts of sulphate reducing bacteria (SRB) related to (A) Truelove and Witts global clinical grade, and (B) sigmoidoscopic grade of mucosal inflammation in patients with ulcerative colitis (Spearman rank correlation test, p<0.0001).
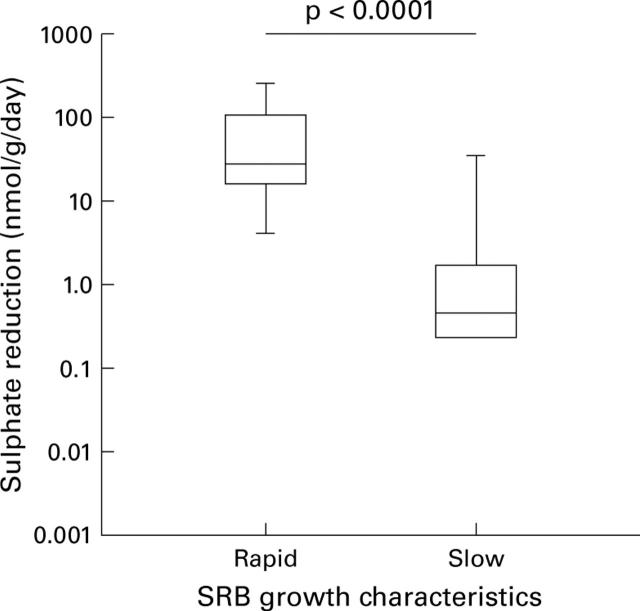
Figure 2 .
Box and whisker plot showing sulphate reduction rates in faeces of all subjects (patients and healthy controls combined) according to colonial growth characteristics of sulphate reducing bacteria (SRB). Rapidly growing SRB are desulfovibrio-like.
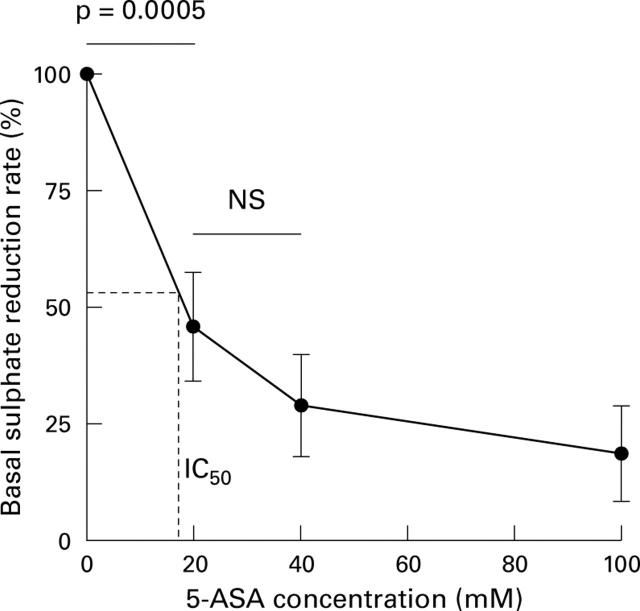
Figure 3 .
Dose response effect of 5-aminosalicylic acid (5-ASA) on dissimilatory sulphate reduction by sulphate reducing bacteria in faecal slurries. Values are mean (SE) for seven healthy subjects selected with high basal sulphate reducing activity.
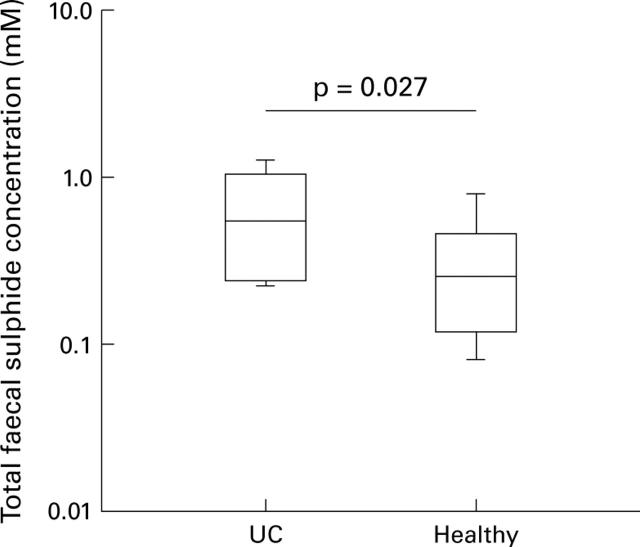
Figure 4 .
Box and whisker plot showing total faecal sulphide concentration in patients with ulcerative colitis (UC) (n=8) not receiving 5-aminosalicylic acid, and healthy controls (n=16).
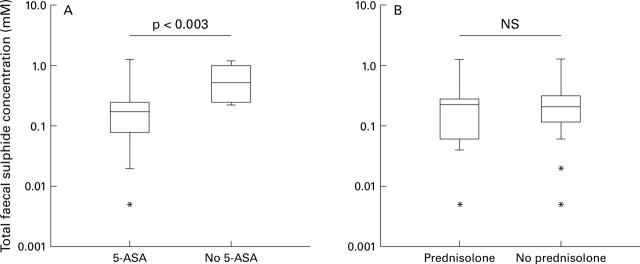
Figure 5 .
Box and whisker plots showing total faecal sulphide concentration in patients with ulcerative colitis (n=37) grouped according to treatment with oral drug therapy. (A) Sulphide concentrations in patients treated with 5-aminosalicylic acid (5-ASA) drugs alone or in combination with prednisolone (n=29) versus no 5-ASA (n=8). (B) Sulphide concentrations in patients treated with prednisolone alone or in combination with 5-ASA (n=11) versus no prednisolone (n=26).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ationu A., Sorensen K., Whitehead B., Singer D., Carter N. Ventricular expression and circulating levels of immunoreactive dynorphin in heart transplant recipients. Clin Sci (Lond) 1993 Jul;85(1):1–4. doi: 10.1042/cs0850001. [DOI] [PubMed] [Google Scholar]
- Azad Khan A. K., Piris J., Truelove S. C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977 Oct 29;2(8044):892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- Breuer R. I., Soergel K. H., Lashner B. A., Christ M. L., Hanauer S. B., Vanagunas A., Harig J. M., Keshavarzian A., Robinson M., Sellin J. H. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997 Apr;40(4):485–491. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M. A., Grahn M. F., Boyle M. A., Hutton M., Rogers J., Williams N. S. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994 Jan;35(1):73–76. doi: 10.1136/gut.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. R., Mortensen P. B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995 Nov;37(5):684–689. doi: 10.1136/gut.37.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short-chain fatty acid enemas in the treatment of distal ulcerative colitis. Eur J Gastroenterol Hepatol. 1997 Feb;9(2):149–153. doi: 10.1097/00042737-199702000-00008. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Bartholomew T. C., Rose F. A., Dodgson K. S. Detoxication of sodium 35 S-sulphide in the rat. Biochem Pharmacol. 1972 Sep 1;21(17):2313–2321. doi: 10.1016/0006-2952(72)90382-6. [DOI] [PubMed] [Google Scholar]
- Devereux R., Delaney M., Widdel F., Stahl D. A. Natural relationships among sulfate-reducing eubacteria. J Bacteriol. 1989 Dec;171(12):6689–6695. doi: 10.1128/jb.171.12.6689-6695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oufir L., Flourié B., Bruley des Varannes S., Barry J. L., Cloarec D., Bornet F., Galmiche J. P. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996 Jun;38(6):870–877. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie I. A., Taylor B. A., Rhodes J. M. Ileal and colonic epithelial metabolism in quiescent ulcerative colitis: increased glutamine metabolism in distal colon but no defect in butyrate metabolism. Gut. 1993 Nov;34(11):1552–1558. doi: 10.1136/gut.34.11.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin T. H. Hydrogen sulphide and total acid-volatile sulphide in faeces, determined with a direct spectrophotometric method. Clin Chim Acta. 1991 Feb 15;196(2-3):127–134. doi: 10.1016/0009-8981(91)90065-k. [DOI] [PubMed] [Google Scholar]
- Florin T., Neale G., Gibson G. R., Christl S. U., Cummings J. H. Metabolism of dietary sulphate: absorption and excretion in humans. Gut. 1991 Jul;32(7):766–773. doi: 10.1136/gut.32.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Dewhirst F. E., Fraser G. J., Paster B. J., Shames B., Murphy J. C. Intracellular Campylobacter-like organism from ferrets and hamsters with proliferative bowel disease is a Desulfovibrio sp. J Clin Microbiol. 1994 May;32(5):1229–1237. doi: 10.1128/jcm.32.5.1229-1237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. L., Zhang W., Singh A., Klurfeld D. M., Don S., Sakata T., Modlin I., Rombeau J. L. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994 Feb;106(2):375–380. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- Gebhart C. J., Barns S. M., McOrist S., Lin G. F., Lawson G. H. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993 Jul;43(3):533–538. doi: 10.1099/00207713-43-3-533. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T., Allison C., Segal I., Vorster H. H., Walker A. R. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990 Jun;31(6):679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Macfarlane G. T., Cummings J. H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988 Aug;65(2):103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. R. Physiology and ecology of the sulphate-reducing bacteria. J Appl Bacteriol. 1990 Dec;69(6):769–797. doi: 10.1111/j.1365-2672.1990.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Plaut A. G., Weinstein L., Patterson J. F., Levitan R. Studies of intestinal microflora. V. Fecal microbial ecology in ulcerative colitis and regional enteritis: relationship to severity of disease and chemotherapy. Gastroenterology. 1968 Apr;54(4):575–587. [PubMed] [Google Scholar]
- Ireland A., Jewell D. P. Mechanism of action of 5-aminosalicylic acid and its derivatives. Clin Sci (Lond) 1990 Feb;78(2):119–125. doi: 10.1042/cs0780119. [DOI] [PubMed] [Google Scholar]
- Lajoie S. F., Bank S., Miller T. L., Wolin M. J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988 Nov;54(11):2723–2727. doi: 10.1128/aem.54.11.2723-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Hansen J., Bytzer P., Bukhave K., Rask-Madsen J. Effects of sulphasalazine and disodium azodisalicylate on colonic PGE2 concentrations determined by equilibrium in vivo dialysis of faeces in patients with ulcerative colitis and healthy controls. Gut. 1984 Nov;25(11):1271–1278. doi: 10.1136/gut.25.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Ellis C. J., Furne J. K., Springfield J., Levitt M. D. Fecal hydrogen sulfide production in ulcerative colitis. Am J Gastroenterol. 1998 Jan;93(1):83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Gibson G. R., Cummings J. H. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992 Jan;72(1):57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Mardini H. A., Lindsay D. C., Deighton C. M., Record C. O. Effect of polymer coating on faecal recovery of ingested 5-amino salicylic acid in patients with ulcerative colitis. Gut. 1987 Sep;28(9):1084–1089. doi: 10.1136/gut.28.9.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Millard S., Babidge W., Rowland R., Roediger W. E. Hydrogen sulphide produces diminished fatty acid oxidation in the rat colon in vivo: implications for ulcerative colitis. Aust N Z J Surg. 1997 May;67(5):245–249. doi: 10.1111/j.1445-2197.1997.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Moore J., Babidge W., Millard S., Roediger W. Colonic luminal hydrogen sulfide is not elevated in ulcerative colitis. Dig Dis Sci. 1998 Jan;43(1):162–165. doi: 10.1023/a:1018848709769. [DOI] [PubMed] [Google Scholar]
- Ohkusa T., Yamada M., Takenaga T., Kitazume C., Yamamoto N., Sasabe M., Takashimizu I., Tamura Y., Sakamoto E., Kurosawa H. [Protective effect of metronidazole in experimental ulcerative colitis induced by dextran sulfate sodium]. Nihon Shokakibyo Gakkai Zasshi. 1987 Oct;84(10):2337–2346. [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979 Jan;32(1):258–265. doi: 10.1093/ajcn/32.1.258. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Hermos J. A., Dzink J. L., Bartlett J. G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology. 1978 Mar;74(3):521–526. [PubMed] [Google Scholar]
- Pitcher M. C., Beatty E. R., Harris R. M., Waring R. H., Cummings J. H. Sulfur metabolism in ulcerative colitis: investigation of detoxification enzymes in peripheral blood. Dig Dis Sci. 1998 Sep;43(9):2080–2085. doi: 10.1023/a:1018867516575. [DOI] [PubMed] [Google Scholar]
- Pitcher M. C., Cummings J. H. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut. 1996 Jul;39(1):1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P., Doré J., Lémann F., Goderel I., Rambaud J. C. Interrelations between populations of methanogenic archaea and sulfate-reducing bacteria in the human colon. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):225–228. doi: 10.1016/0378-1097(92)90160-p. [DOI] [PubMed] [Google Scholar]
- Pullan R. D., Thomas G. A., Rhodes M., Newcombe R. G., Williams G. T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994 Mar;35(3):353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Decreased sulphur aminoacid intake in ulcerative colitis. Lancet. 1998 May 23;351(9115):1555–1555. doi: 10.1016/s0140-6736(05)61120-8. [DOI] [PubMed] [Google Scholar]
- Roediger W. E., Duncan A., Kapaniris O., Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology. 1993 Mar;104(3):802–809. doi: 10.1016/0016-5085(93)91016-b. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980 Oct 4;2(8197):712–715. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Ruddell W. S., Dickinson R. J., Dixon M. F., Axon A. T. Treatment of distal ulcerative colitis (proctosigmoiditis) in relapse: comparison of hydrocortisone enemas and rectal hydrocortisone foam. Gut. 1980 Oct;21(10):885–889. doi: 10.1136/gut.21.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Jewell D. P., Rosenberg W. M., Bell J. I. Genetics of inflammatory bowel disease. Gut. 1994 May;35(5):696–700. doi: 10.1136/gut.35.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal I., Walker A. R., Lord S., Cummings J. H. Breath methane and large bowel cancer risk in contrasting African populations. Gut. 1988 May;29(5):608–613. doi: 10.1136/gut.29.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk Laursen L., Stokholm M., Bukhave K., Rask-Madsen J., Lauritsen K. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut. 1990 Nov;31(11):1271–1276. doi: 10.1136/gut.31.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strocchi A., Ellis C. J., Furne J. K., Levitt M. D. Study of constancy of hydrogen-consuming flora of human colon. Dig Dis Sci. 1994 Mar;39(3):494–497. doi: 10.1007/BF02088333. [DOI] [PubMed] [Google Scholar]
- Strocchi A., Ellis C. J., Levitt M. D. Use of metabolic inhibitors to study H2 consumption by human feces: evidence for a pathway other than methanogenesis and sulfate reduction. J Lab Clin Med. 1993 Feb;121(2):320–327. [PubMed] [Google Scholar]
- Strocchi A., Furne J. K., Ellis C. J., Levitt M. D. Competition for hydrogen by human faecal bacteria: evidence for the predominance of methane producing bacteria. Gut. 1991 Dec;32(12):1498–1501. doi: 10.1136/gut.32.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strocchi A., Furne J., Ellis C., Levitt M. D. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut. 1994 Aug;35(8):1098–1101. doi: 10.1136/gut.35.8.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strocchi A., Levitt M. D. Factors affecting hydrogen production and consumption by human fecal flora. The critical roles of hydrogen tension and methanogenesis. J Clin Invest. 1992 Apr;89(4):1304–1311. doi: 10.1172/JCI115716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tragnone A., Valpiani D., Miglio F., Elmi G., Bazzocchi G., Pipitone E., Lanfranchi G. A. Dietary habits as risk factors for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995 Jan;7(1):47–51. [PubMed] [Google Scholar]
- Travis S. P., Jewell D. P. Salicylates for ulcerative colitis--their mode of action. Pharmacol Ther. 1994 Aug;63(2):135–161. doi: 10.1016/0163-7258(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Tsai H. H., Dwarakanath A. D., Hart C. A., Milton J. D., Rhodes J. M. Increased faecal mucin sulphatase activity in ulcerative colitis: a potential target for treatment. Gut. 1995 Apr;36(4):570–576. doi: 10.1136/gut.36.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. H., Sunderland D., Gibson G. R., Hart C. A., Rhodes J. M. A novel mucin sulphatase from human faeces: its identification, purification and characterization. Clin Sci (Lond) 1992 Apr;82(4):447–454. doi: 10.1042/cs0820447. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Pinkus L. M., Jakoby W. B. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980 Oct 15;29(20):2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- van Hees P. A., Bakker J. H., van Tongeren J. H. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut. 1980 Jul;21(7):632–635. doi: 10.1136/gut.21.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]