Abstract
In vitro, polynucleotide phosphorylase of Escherichia coli can both synthesize RNA by using nucleotide diphosphates as precursors and exonucleolytically degrade RNA in the presence of inorganic phosphate. However, because of the high in vivo concentration of inorganic phosphate in exponentially growing cells, it has been assumed that the enzyme works exclusively as an exonuclease. Here we demonstrate that, contrary to this prediction, polynucleotide phosphorylase not only synthesizes long, highly heteropolymeric tails in vivo, but also accounts for all of the observed residual polyadenylylation in poly(A) polymerase I deficient strains. In addition, the enzyme is responsible for adding the C and U residues that are found in poly(A) tails in exponentially growing cultures of wild type E. coli.
Polyadenylylation in Escherichia coli has been shown to play a significant role in RNA decay (1–5). Although these results suggest that the posttranscriptional addition of poly(A) tails is an important feature of RNA metabolism in the bacterium, a number of important unanswered questions remain. One of these questions relates to the nature of the residual polyadenylylation in strains deficient in poly(A) polymerase (PAP) I. Although several experimental approaches have shown that PAP I accounts for between 90 and 95% of the poly(A) tails in wild-type E. coli (3, 5), there have been reports of a second PAP (6). Because a recent analysis of the f310 gene has demonstrated that it does not encode a PAP (7), it still is not clear which enzyme is responsible for poly(A) tail synthesis in a PAP I− (ΔpcnB) mutant.
In addition, sequence analysis of poly(A) tails isolated from exponentially growing cells (5), bacteriophage T7 infected cells (8), or stationary phase cultures (9) has detected the presence of occasional C, U, and G residues, a phenomenon not observed with nuclear encoded eukaryotic mRNAs. Although several laboratories have demonstrated that E. coli PAP I will incorporate C, U, and G at reduced frequencies in vitro (10, 11), it has not yet been determined whether the enzyme acts similarly in vivo.
E. coli does contain three additional enzymes that theoretically could serve as a PAP. These are polynucleotide phosphorylase (PNPase; pnp), RNase PH (rph), and tRNA nucleotidyltransferase (cca). In fact, analysis at the amino acid level of a number of these proteins along with PAPs has identified a series of consensus motifs that have suggested either a common evolutionary origin or that one activity may have given rise to the other to form a large superfamily (12).
PNPase and RNase PH are both reversible enzymes that can degrade RNA by using inorganic phosphate or synthesize RNA by using any nucleotide diphosphate as a precursor (13–15). However, it has always been assumed that, because of the high in vivo concentration of inorganic phosphate (10 mM) in the bacterium (16), PNPase and RNase PH will work only degradatively. In fact, PNPase has been shown to be an important component of the mRNA decay system (17, 18). In the case of tRNA nucleotidyltransferase, it normally functions in the repair of the 3′ end of tRNAs by adding a single A moiety (19, 20). However, there is no evidence that it can synthesize poly(A) tails.
To help resolve these issues, we have determined total poly(A) levels in strains containing varying amounts of PAP I, PNPase, RNase PH, and tRNA nucleotidyltransferase. In addition, we cloned and sequenced poly(A) tails associated with lpp, rpsO, and 23S rRNA transcripts. Our data demonstrate that PNPase accounts for both residual polyadenylylation in the absence of PAP I and the incorporation of non-A residues into poly(A) tails in wild-type E. coli. The results presented provide insights into the mechanism of PNPase action as well as the importance of polyadenylylation in the decay of mRNAs.
Materials and Methods
Bacterial Strains and Plasmids.
The E. coli strains used in this study were all derived from MG1693 (rph-1 thyA715) provided by B. Bachmann (E. coli Genetic Stock Center, Yale University). SK7988 (ΔpcnB rph-1 thyA715; ref. 3) and SK9129 (pnp-7 rph-1 thyA715/pBMK11; ref. 21) have been described. SK6699 (ΔpcnB rph-1 pnp-7) was constructed from SK5691 (pnp-7 rph-1 thyA715; ref. 22), and SK9166 (ΔpcnB pnp-7 cca∷cm rph-1 thyA715) was generated from SK6699 by using bacteriophage P1-mediated transduction. Plasmids pKAK7 (Apr pnp+; ref. 23), pPP1 (Apr rph+; ref. 24), and pBMK11 (cmr pcnB+; ref. 5) have been described. Plasmid pEC7 (Apr cca+) is very similar to plasmid pEC4 (Apr cca+; ref. 25), which contains a 2-kilobase insert carrying the cca gene cloned into the SalI and BamHI sites of pUC13. pWSK29 (26) was used to clone and sequence cDNAs.
Preparation and Analysis of Total RNA.
Bacterial strains were grown with shaking at 37°C in Luria broth supplemented with thymine (50 μg/ml). When necessary chloramphenicol (20 μg/ml) and ampicillin (50 μg/ml) were added to the medium. Expression of the pcnB gene in pBMK11 was induced by adding 350 μM isopropyl β-d-thiogalactoside (United States Biochemical) when cell cultures reached Klett 50 (≈1 × 108 cells per ml). At selected times, total RNA from the cells was isolated and analyzed on dot blots, as described by O'Hara et al. (3), by spotting 5 μg of total RNA onto Magnacharge nylon membranes (Micron Separations, Westboro, MA) and hybridizing to either 32P-labeled oligo(dT)20 or oligo(dT)30. Poly(A) tail-sizing assays were carried out as described by O'Hara et al. (3).
Reverse Transcription–PCR Cloning and Sequencing of cDNA.
All reverse transcriptions were carried out by using Superscript reverse transcriptase (BRL). An oligo (dT) adapter primer [AP, 5′-GATGGTACCTCTAGAGCTC(T)17-3′] containing a multiple cloning site (MCS; underlined) was used to reverse transcribe poly(A)-tailed RNA by using total RNA (1–10 μg). In the case of SK6699 (ΔpcnB pnp-7 rph-1), a second approach was also used to obtain the 3′ sequences of individual RNA transcripts that was independent of poly(A) tail-dependent reverse transcription. The RNA (1 μg) was ligated to primer AAP-REV (5′-GAGCTCTAGAGGTACCATC-3′) at the 3′ ends by using 20 units of T4 RNA ligase (NEB) in a 10-μl reaction volume (27). A second primer AAP (5′-GATGGTACCTCTAGAGCTC-3′) complementary to primer AAP-REV was used for cDNA synthesis of the ligation products. The primer AAP, which was also homologous to the MCS of the primer AP, was used as the 3′ primer to amplify both AP- and AAP-primed cDNA (5 μl) along with a gene-specific primer at the 5′ end. The 5′ gene-specific primer was designed to amplify the entire coding sequences of lpp, ≈200–280 nt of rpsO and ≈425 nt of 23S rRNA from the 3′ ends. The PCR products were purified, analyzed on agarose gels, cloned into pWSK29, and transformed into E. coli DH5α by using standard techniques. All DNA sequencing was carried out manually by using the fmol Sequencing Kit from Promega.
Western Blotting.
Western blotting of PNPase was carried out essentially as described (21).
Results
Effect of PNPase, RNase PH, and tRNA Nucleotidyltransferase on Poly(A) Levels in a PAP I-Deficient Strain.
Previous experiments have shown that PAP I accounts for over 90% of the poly(A) tails in E. coli (3, 5). However, based on RNA-DNA dot blots using an oligo (dT)20 probe, there is only about a 75% reduction in the relative poly(A) level in a ΔpcnB mutant [compare MG1693 (pcnB+) and SK7988 (ΔpcnB) in Table 1; ref. 7]. As a first approach to determining which enzyme(s) account for the residual polyadenylylation, we examined the relative poly(A) levels in a series of strains carrying combinations of mutations in pcnB (PAP I), pnp (PNPase), cca (tRNA nucleotidyltransferase), and rph (RNase PH; Table 1). All of the strains carried the rph-1 allele, because MG1693 contains a frameshift mutation in this gene (28). Surprisingly, the ΔpcnB pnp-7 rph-1 triple mutant (SK6699) exhibited a small increase in poly(A) compared with the ΔpcnB rph-1 double mutant (SK7988; Table 1). Inactivation of the tRNA nucleotidyltransferase (cca∷cam) in SK6699 (ΔpcnB pnp-7 rph-1) led to a slow growing quadruple mutant (SK9166, ΔpcnB pnp-7 cca∷cam rph-1) in which the poly(A) level was less than that observed in the ΔpcnB rph-1 double mutant (SK6699; Table 1).
Table 1.
Poly(A) levels in various genetic backgrounds*
| Strain | Genotype | Pixels × 104
|
|
|---|---|---|---|
| Hybridization probe
| |||
| (dT)20† | (dT)30‡ | ||
| MG1693 | rph-1§ | 12 ± 2 | 3 ± 1 |
| SK7988 | ΔpcnB rph-1 | 3 ± 1 | 0.4 ± 0.2 |
| SK6699 | ΔpcnB pnp-7 rph-1 | 5 ± 1 | 0.6 ± 0.2 |
| SK9166 | ΔpcnB pnp-7 cca∷cam rph-1 | 2 ± 1 | 0.6 ± 0.2 |
| SK9161 | ΔpcnB rph-1/pKAK7 (pnp+) | 10 ± 2 | 1 ± 0.2 |
| Control¶ | 0.4 ± 0.2 | 0.4 ± 0.2 | |
Pixel values corresponding to the amount of 32P-labeled oligo(dT) probe that hybridized to total RNA isolated from each strain listed as determined with a Model 400 Molecular Dynamics PhosphorImager directly represent the amount of poly(A) present. Each value is the average of at least five or six independent experiments.
Hybridization temperature of 30°C.
Hybridization temperature of 50°C.
Jensen (28) has demonstrated that the progenitor of MG1693, MG1655, contains a frameshift mutation in rph.
No RNA spotted on the hybridization membrane.
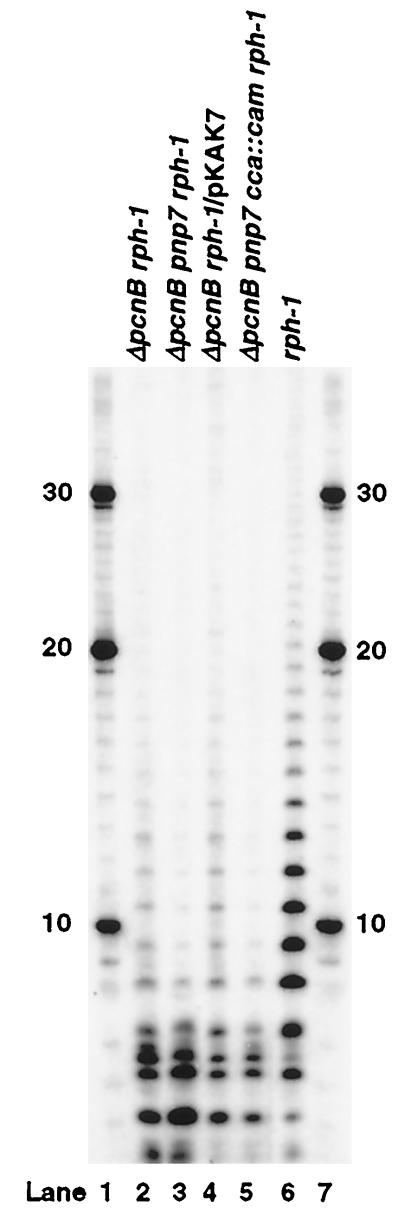
To help determine whether PNPase or tRNA nucleotidyltransferase was responsible for the residual polyadenylylation observed in a PAP I− strain, we performed a poly(A) sizing assay to determine the length of the poly(A) tails in each genetic background. Poly(A) tails of primarily less that 15 nt were observed in SK7988 (ΔpcnB rph-1; Fig. 1, lane 2) compared with tails of up to 40 nt in the MG1693 (rph-1) control (Fig. 1, lane 6). In the ΔpcnB pnp-7 rph-1 triple mutant (SK6699), the length of the poly(A) tails was reduced further to less than 8 nt with a slight increase in the amount of 2- to 7-nt poly(A) species (Fig. 1, lane 3). Interestingly, in the quadruple mutant (ΔpcnB pnp-7 cca∷cam rph-1; SK9166), the pattern was similar to that observed with SK6699 (ΔpcnB pnp-7 rph-1) except that there was a reduction in the amount of poly(A) species in the 3- to 5-nt range (Fig. 1, lanes 3 and 5).
Figure 1.
Comparison of poly(A) tails in various strains. All RNA samples (20 μg per lane) from exponentially growing cultures were processed for the poly(A) sizing assay on a 20% polyacrylamide gel. Lane 2, SK7988; lane 3, SK6699; lane 4, SK9161; lane 5, SK9166; lane 6, MG1693; lanes 1 and 7, 32P-labeled oligo d(A) size standards (nt) as marked. The genotype of each strain is as noted.
Overexpression of tRNA Nucleotidyltransferase Does Not Increase Poly(A) Levels.
tRNA nucleotidyltransferase is known to add a single A residue to tRNAs or tRNA-like substrates under normal circumstances (29). To test whether this enzyme is involved in polyadenylylation, we transformed MG1693 (rph-1), SK7988 (ΔpcnB rph-1), SK6699 (ΔpcnB pnp-7 rph-1), and SK9166 (ΔpcnB pnp-7 cca∷cam rph-1) with pEC7, a ColE1-based plasmid carrying the wild-type cca gene. Increased tRNA nucleotidyltransferase activity did not lead to any significant change in total poly(A) levels in any of the genetic backgrounds tested (data not shown).
Overexpression of PNPase Leads to Increased Levels of Polyadenylylation in PAP I-Deficient Strains.
Although there was a small increase in the poly(A) level in the ΔpcnB pnp-7 rph-1 triple mutant (SK6699) compared with that in the ΔpcnB rph-1 double mutant (SK7988; Table 1), the poly(A) sizing assay (Fig. 1, lanes 2 and 3) showed that the difference in the poly(A) levels was probably caused by an increase in the number of very short poly(A) sequences (3–7 nt) in SK6699. Because it has been shown that PNPase can play a direct role in poly(A) degradation (5, 21), the increase in poly(A) level in SK6699 (ΔpcnB pnp-7 rph-1) could have resulted solely from the absence of this activity. However, if that were the case, we would have expected to see an increase in the number of poly(A) tails that were longer than 8 nt. In fact, the amount of longer poly(A) tails was significantly reduced in SK6699 (ΔpcnB pnp-7 rph-1) compared with that in SK7988 (ΔpcnB rph-1), suggesting that PNPase might in fact be responsible for their synthesis.
Accordingly, we determined whether overproducing PNPase in a ΔpcnB rph-1 (SK7988) mutant led to any significant increase in the observed level of polyadenylylation. Interestingly, after transformation of SK7988 with pKAK7, a ColE1-derived plasmid carrying the wild-type PNPase gene (SK9161; ref. 23), the poly(A) level increased to almost wild-type levels (MG1693, Table 1). A poly(A) sizing assay on RNA isolated from SK9161 (ΔpcnB rph-1/pKAK7; Fig. 1, lane 4) showed both an increase in the amount and the length of the poly(A) tails compared with those of both SK6699 (ΔpcnB pnp-7 rph-1; Fig. 1, lane 3) and SK7988 (ΔpcnB rph-1; Fig. 1, lane 2). In fact, a quantitative comparison of lanes 2 and 4 with a PhosphorImager showed a 3-fold increase in poly(A) tails in SK9161 compared with SK7988. Similar increases in poly(A) were also observed in the triple (Δpcnb pnp-7 rph-1) and quadruple (Δpcnb pnp-7 ccs∷cam rph-1) mutants after transformation with pKAK7 (data not shown).
PNPase Synthesizes Highly Heteropolymeric Tails in Vivo in the Absence of PAP I.
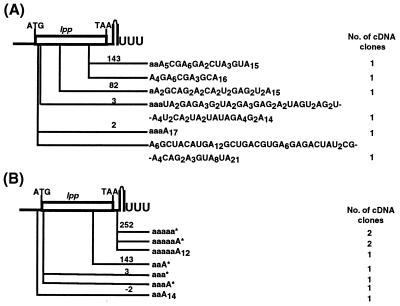
Because the results described above indicated that PNPase could significantly alter both the level of polyadenylylation and the length of the tails in the absence of PAP I, we analyzed the composition of poly(A) tails associated with the lpp transcript. This mRNA was chosen because we have previously shown that a small fraction (0.12%) of this transcript still is polyadenylylated in the absence of PAP I (5). In addition, we hypothesized that if PNPase were responsible for the poly(A) tails observed in a PAP I− strain, they should be highly heteropolymeric because the enzyme incorporates any of the four nucleotide diphosphates randomly into a oligoribonucleotide primer (30). When we sequenced reverse transcription–PCR clones of lpp cDNAs derived from SK7988 (ΔpcnB rph-1), five of six of the independent isolates had highly heteropolymeric tails that contained A > G > U > C (Fig. 2A). In addition, in contrast to the cDNAs isolated from a PAP I+ strain (5), none of the tails occurred after the transcription terminator.
Figure 2.
Sites and composition of lpp poly(A) tails isolated from strains deficient in either PAP I or PAP I and PNPase. (A) SK7988 (ΔpcnB rph-1). (B) SK6699 (ΔpcnB pnp-7 rph-1). All of the poly(A) sequences were obtained from AP-primed cDNA except that ones marked with (*) in B, which were obtained from AAP-primed cDNA as described in Materials and Methods. The letter a in the poly(A) sequences indicates a chromosomally encoded nucleotide. The position (not drawn to scale) of each or set of poly(A) tails marked (nt) is from the translation start site.
To prove that these tails had been synthesized by PNPase, we isolated cDNAs from a ΔpcnB pnp-7 rph-1 mutant (SK6699). Because the poly(A) tails seemed to be very short in this strain (Fig. 1, lane 3), we used two distinct approaches for the reverse transcription of the RNA. In the first approach, total RNA was used as the template to reverse transcribe the poly(A) tailed RNA by using an oligo(dT) primer (AP; ref. 5). With the second technique, an oligonucleotide (AAP-REV) was ligated to the 3′ ends of the RNA by using RNA ligase (27). Reverse transcription was then carried out by using a primer complementary (AAP) to the ligated oligonucleotide.
As shown in Fig. 2B, two types of sequences were obtained for the lpp cDNAs. With the AP-primed reverse transcription, short poly(A) tails that had been added onto internally encoded stretches of A were detected. With the AAP-mediated reverse transcription approach, only a single posttranscriptionally added A residue was observed, again located at internally encoded stretches of A residues. The addition of these single A residues probably arose from the action of tRNA nucleotidyltransferase. It should be noted that when the AP primer was used, 10-fold more template was used both for the reverse transcription step and the PCR amplification of lpp cDNA from SK6699 compared with that of SK7988. In addition, with the ligation-mediated reverse transcription method, the majority of the cloned sequences did not have any posttranscriptionally added nucleotide sequences at the 3′ end (data not shown).
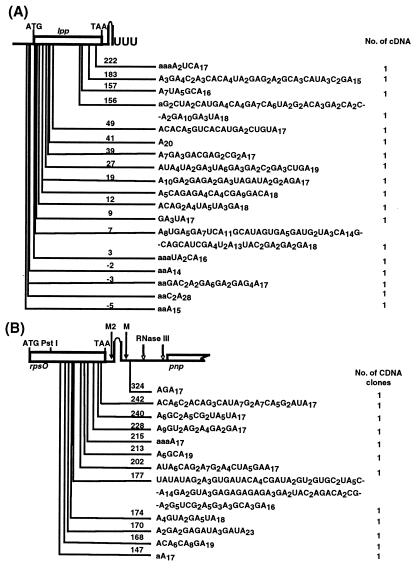
As a further test to show that PNPase was responsible for the heteropolymeric tails observed in SK7988 (ΔpcnB rph-1), we isolated and sequenced both lpp and rpsO cDNAs from SK9161 (ΔpcnB rph-1/pKAK7 [pnp+]), a strain in which the enzyme was overproduced. As shown in Fig. 3 A and B, with both lpp (15/18) and rpsO (10/12), 83% of the tails were highly heteropolymeric. None of the tails in either transcript was added after the Rho independent terminator, the predominant location in PAP I+ E. coli (31, 5). In addition, some of the tails were over 130 nt in length, far longer than any of the tails isolated from PAP I+ E. coli (5).
Figure 3.
Sites and composition of lpp and rpsO poly(A) tails isolated from SK9161 (ΔpcnB rph-1/pKAK7). (A) lpp. (B) rpsO. Poly(A) addition sites are as described in Fig. 2. M and M2 are RNase E cleavage sites as previously described (31).
Interestingly, all of the heteropolymeric poly(A) sequences in Figs. 2A and 3 had Gs, Cs, and Us mainly toward the 5′ ends of the tails, an observation similar to that seen in heteropolymeric tails isolated from wild-type E. coli (5). Although the exact reason for such differential polymerization is not clear, it should be noted that all of the poly(A) tails in Figs. 2A and 3 were obtained from AP-primed cDNA, which insures that the 3′ ends will contain a homopolymeric stretch of A residues. When cDNAs were isolated by using the AAP-primed cDNA method, heteropolymeric tails without long stretches of As at the 3′ end were also obtained (data not shown).
PNPase Is Responsible for Generating Heteropolymeric Tails in PAP I+ E. coli.
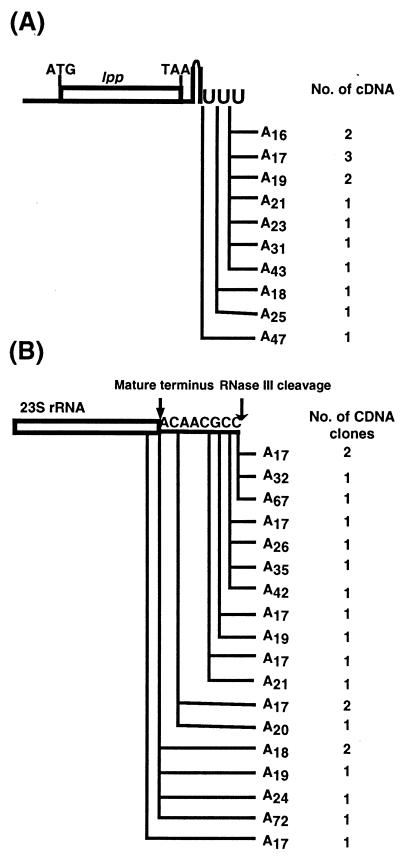
We have previously shown that ≈10% of the poly(A) tails in exponentially growing PAP I+ E. coli contain occasional C and U residues, increasing to over 30% in the presence of increased amounts of PAP I (5). To determine whether the C and U residues observed in these tails arose from misincorporation by PAP I as previously thought or from the action of PNPase, we sequenced poly(A) tails isolated from a pnp-7/pBMK11(pcnB+) strain (SK9129). We chose this strain because, in this genetic background, there is five times more poly(A) than observed under similar induction conditions in a wild-type control (21). Under these circumstances, we expected high levels of heteropolymeric tails if they resulted from the action of PAP I. Surprisingly, all of the independently derived lpp cDNAs (14/14) and 23S rRNA cDNAs (21/21) were homopolymeric and ranged in length from 17 to 72 residues (Fig. 4 A and B). In addition, the polyadenylylation sites closely resembled those obtained in the wild-type strain (5) but not those seen in Fig. 2 and 3. None of the tails were as long as some of those obtained from SK7988 (ΔpcnB rph-1; Fig. 2A) or SK9161 (ΔpcnB rph-1/pKAK7 [pnp+]; Fig. 3).
Figure 4.
Sites and composition of lpp and 23S rRNA poly(A) tails isolated from SK9129 (pnp-7 rph-1/pMBK11[pcnB+]). (A) lpp. (B) 23S rRNA. PAP I was induced for 15 min in the presence of 350 μM isopropyl β-d-thiogalactoside ITPG (21). Poly(A) addition sites are as described in Fig. 2.
The Percentage of Heteropolymeric Tails Is Related to in Vivo PNPase Level.
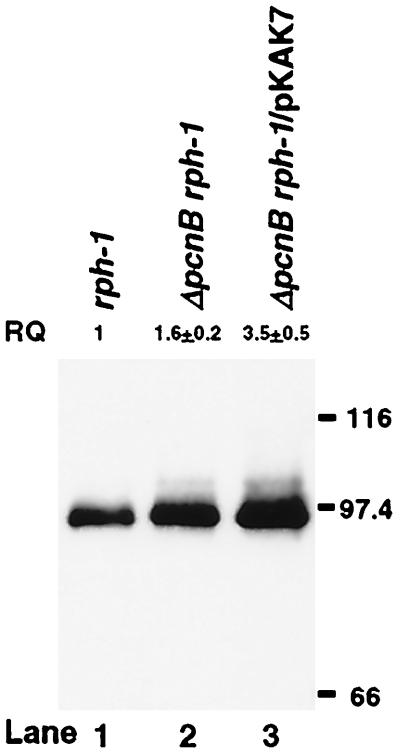
Because the absence of PNPase (SK6699 and SK9129) seemed to be responsible for the elimination of heteropolymeric tails (Figs. 2B and 4), we hypothesized that strains (SK7988 and SK9161) containing a higher percentage of heteropolymeric tails (Figs. 2A and 3) would also have increased levels of PNPase. As expected, a Western blot demonstrated a 1.6-fold increase of PNPase protein in SK7988 (ΔpcnB rph-1) and a 3.5-fold increase in SK9161 (ΔpcnB rph-1/pKAK7) compared with MG1693 (rph-1; Fig. 5, lanes 1–3). A similar increase in PNPase levels and the percentage of heteropolymeric tails has been reported after induction of PAP I in wild-type E. coli (5, 21).
Figure 5.
Measurement of PNPase levels in various strains. Cell extracts (5 μg per lane) from exponentially growing cultures of MG1693 (lane 1), SK7988 (lane 2), and SK9161 (lane 3) were separated on an SDS/8% polyacrylamide gel, transferred to a poly(vinylidene difluoride) nylon membrane, and probed with PNPase antibody. The genotype of each strain is noted at the top of each lane. Protein molecular mass standards (kDa, Bio-Rad) are marked at the right of the blot. RQ, relative quantity, determined by densitometric quantitation represents the average of three to five independent experiments. The protein level of MG1693 was set at 1.
Poly(A) Sequences Observed in a ΔpcnB pnp-7 rph-1 Triple Mutant Are Internally Encoded.
If the residual polyadenylylation observed in the absence of PAP I is carried out by PNPase, how can the increased poly(A) levels in SK6699 (ΔpcnB pnp-7 rph-1; Table 1) be explained? Because quantitation of the poly(A) sizing assay (Fig. 1) suggested that the increase in polyadenylylation in SK6699 (ΔpcnB pnp-7 rph-1) arose from changes in the distribution and amount of short sequences in the 3- to 7-nt range (Fig. 1, lane 3), we suspected that we were probably detecting short poly(A) sequences that were initially transcribed as part of various mRNAs. In a PAP I− strain, such sequences often served as polyadenylylation sites for PNPase (Figs. 2A and 3). In the absence of both PAP I and PNPase, these sequences would be stabilized such that they would be labeled by 32pCp and RNA ligase. In fact, a search of the complete E. coli genome by using GCG's find patterns program yielded more than 35,000 poly(A) sequences ranging from 5 to 15 nts (data not shown), some of which would be expected to hybridize to an oligo(dT)20 primer in the dot blot experiment described in Table 1.
Accordingly, we carried out a series of RNA-DNA dot blots by using RNA isolated from rph-1 (MG1693), ΔpcnB rph-1 (SK7988), ΔpcnB pnp-7 rph-1 (SK6699), ΔpcnB pnp-7 cca∷cam rph-1 (SK9166), and ΔpcnB pnp-7 rph-1/pKAK7 (SK9161) strains and either oligo(dT)20 or oligo(dT)30 as probes. With the oligo(dT)30 probe, the hybridization temperature was increased from 30°C to 50°C. As was expected, there was a general reduction in the level of hybridization with all of the strains when the oligo(dT)30 probe was used because of the increased stringency (Table 1). Moreover, under these conditions, the level of hybridization observed with RNA isolated from SK7988 (ΔpcnB rph-1), SK6699 (ΔpcnB pnp-7 rph-1), SK9165 (ΔpcnB pnp-7 cca∷cam rph-1), and SK9166 (ΔpcnB pnp-7 cca∷cam rph-1) was not significantly higher than background (Table 1). These results corroborated the idea that the increased level of apparent poly(A) tails in SK6699 compared with SK7988 (Table 1) arose from the detection of short poly(A) sequences contained within transcripts.
Overexpression of RNase PH Leads to Reduced Poly(A) Levels.
Along with PNPase, RNase PH is a second E. coli exoribonuclease that catalyzes phosphate-dependent RNA degradation (32) and can synthesize RNA in vitro by using nucleotide diphosphates as precursors (33). Although the primary function of RNase PH is thought to be in processing the 3′ ends of tRNAs (34, 35), we decided to determine whether, in vivo, this enzyme might play a role in polyadenylylation. All of the strains described in Table 1 carry the rph-1 mutation, an allele that has been shown to quantitatively inactivate the 3′ → 5′ exonuclease activity of RNase PH (15). However, because the rph-1 mutation is in the carboxyl-terminal region of the protein, we decided to rule out any role for RNase PH in polyadenylylation by transforming MG1693 (rph-1), SK7988 (ΔpcnB rph-1), SK6699 (ΔpcnB pnp-7 rph-1), and SK9166 (ΔpcnB pnp-7 cca∷cam rph-1) with pPP1, a ColE1-derived plasmid carrying the wild-type copy of the rph gene (24). Overproduction of RNase PH led to reduction of poly(A) levels in all of the genetic backgrounds tested (data not shown).
Discussion
In this report, we have examined the possibility that PNPase, RNase PH, or tRNA nucleotidyltransferase, acting in concert or by themselves, could account for the residual polyadenylylation observed in a PAP I− mutant. Although PNPase was the most likely candidate for being the backup PAP because of the highly reversible nature of its reaction mechanism (30), the high intracellular level of inorganic phosphate in E. coli (16) has led to the assumption that the enzyme functions exclusively in vivo as an exoribonuclease. However, the data presented in Table 1 and Figs. 1–4 clearly demonstrate that, in vivo, PNPase also functions as a PAP. Thus, all of the heteropolymeric tails observed in SK7988 (ΔpcnB rph-1; Fig. 2A) disappeared in the ΔpcnB pnp-7 rph-1 strain (SK6699; Fig. 2B).
Furthermore, previous work has demonstrated the presence of non-A residues in poly(A) tails observed in exponentially growing cultures (5), stationary phase cultures (9), and bacteriophage T7-infected strains of E. coli (8). Although it has been presumed that these heteropolymeric tails arise from misincorporation of C, U, and G residues by PAP I, it would seem that the occasional C and U residues observed in exponentially growing cultures (5) arise from the action of PNPase, not PAP I (Fig. 4). The composition of the tails observed in stationary phase cultures (9) and T7-infected E. coli (8) suggests that PNPase may also be responsible for their synthesis. Taken together, these results indicate that despite the overall high intracellular concentration of inorganic phosphate, PNPase accounts both for the residual polyadenylylation observed in a ΔpcnB mutant and the incorporation of non-A residues into poly(A) tails in wild-type cells.
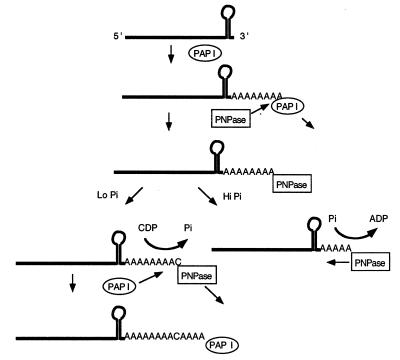
The demonstration of in vivo biosynthetic activity for PNPase suggests that, in wild-type E. coli, both PAP I and PNPase can be involved in the synthesis of the same poly(A) tail (Fig. 6). There is already direct evidence that these two enzymes compete for a 3′ terminus either to continue synthesis (PAP I) or to initiate degradation (PNPase; refs. 5 and 21). In addition, it has been shown that, in vitro, PNPase can work either biosynthetically or degradatively, depending on the inorganic phosphate concentration (14). Thus, it is possible that if inorganic phosphate levels are reduced transiently, PNPase can add one or more nucleotides rather than degrading the poly(A) tail (Fig. 6). After PNPase disassociation, the terminus would become available either for PAP I to continue biosynthesis or for the binding of an exonuclease such as RNase II. Because it is not possible to distinguish an A residue added by PNPase from one polymerized by PAP I, it is not clear how extensive the PNPase polymerization reaction is in a PAP I+ stain. However, one intriguing feature of this process is that in the presence of PAP I, PNPase seems to add C and U residues preferentially (5). In contrast, when PNPase is working in the absence of PAP I, G is the most prevalent residue after A (Figs. 2A and 3 A and B).
Figure 6.
Schematic representation of the role of PNPase in poly(A) tail metabolism in wild-type E. coli. After primary polyadenylylation of the transcript by PAP I, PNPase may bind to the 3′ end of the poly(A) tail. Depending on the availability of inorganic phosphate (Pi), PNPase works either degradatively or biosynthetically. In the presence of high Pi concentration, it degrades the poly(A) tail releasing diphosphates. If the Pi concentration is low, it works biosynthetically adding one or more nucleotides to the existing poly(A) tail and in the process generates inorganic phosphate. On dissociation, the 3′ end again is available to PAP I for further polymerization.
Interestingly, the reaction mechanism associated with PNPase degradation of RNA may provide a clue into how the inorganic phosphate level can temporarily be reduced sufficiently to permit biosynthesis to occur. Specifically, one phosphate moiety is required for each phosphodiester bond cleaved (Fig. 6). Because PNPase is known to have a high turnover number, extensive degradation of a polyadenylylated mRNA transcript could lead to a transient but large drop in the phosphate concentration within a limited microenvironment. In the presence of nucleotide diphosphates, biosynthesis could then take place. However, because the biosynthetic reaction releases inorganic phosphate (Fig. 6), one would expect the increase in free phosphate to limit rapidly the extent of the biosynthetic reaction. This type of transient change in phosphate concentration might be expected to occur more easily if the degradation and/or polyadenylylation of a transcript occurred at a fixed location, for example, attached to the inner membrane.
The difference in the profile of polyadenylylation sites between PAP I− strains (SK7988, SK6699, and SK9161; Figs. 1–3) and the PAP I+ control (31, 5; Fig. 4) indicates an important role for polyadenylylation in normal mRNA decay. In a PAP I+ strain, most of the poly(A) tails that have been isolated (Fig. 4; ref. 5) occurred after a Rho-independent transcription terminator, presumably facilitating the efficient degradation of the entire transcript through a combination of endonucleolytic and exonucleolytic steps, without generating any significant level of stable decay intermediates. In contrast, in a PAP I− strain, the heteropolymeric tails generated by PNPase were distributed throughout the coding sequences (Figs. 2A and 3), possibly resulting from the less efficient degradation of these RNA molecules in the absence of PAP I.
It has been hypothesized that poly(A) tails provide the basis for the binding of multiprotein RNA degrading complexes such as the RNase E-based degradosome (36). Although no in vitro difference was observed between a synthetic homopolymeric and a heteropolymeric tail in stimulating the degradation of a specific substrate by the degradosome (37), in vivo, it seems that the heteropolymeric tails synthesized in a PAP I− strain may not be a suitable substrate with which the degradosome can interact. This conclusion is supported by the observation that increased poly(A) levels generated by PAP I enhanced mRNA decay rates (5), whereas mRNA half-lives increased in PAP I− strains (3, 5). In addition, the significant differences in the location of the poly(A) addition sites in the presence or absence of PAP I (ref. 5; Figs. 2–4) also support the hypothesis of a significant change in the mechanism of transcript degradation. However, the heteropolymeric tails synthesized by PNPase may enhance the degradation of mRNA decay intermediates that have significant secondary structures at their 3′ termini and would otherwise be very stable in the absence of an extended unstructured region.
Finally, it is interesting that the level of PNPase increases in the absence of PAP I (Fig. 5, lane 2). Although this increase may simply be caused by increased stability of the pnp transcript in a ΔpcnB mutant, it could also be related to the need for more enzyme for degrading mRNAs. It should be noted that although the PAP I− PNPase− strain (SK6699, ΔpcnB pnp 7rph-1) grows considerably more slowly than the PAP I− strain (SK7988, ΔpcnB rph-1), it still is viable. Thus, on the surface, it would seem that in the complete absence of polyadenylylation the cell can still survive. However, even though the pnp-7 allele is a nonsense mutation (R. D. Ivarie and S.R.K., unpublished results), Western blot analysis has revealed 2–4% of wild-type PNPase levels in such mutants.
Acknowledgments
Plasmids pPP1 and pEC7 and the caa∷cam allele were provided by M. Deutscher (Department of Biochemistry, University of Miami School of Medicine, Miami). PNPase antibodies were obtained from A. J. Carpousis (Centre National de la Recherche Scientifique, Toulouse, France). This work was supported by National Institutes of Health Grants GM28760 and GM57220 to (S.R.K.).
Abbreviations
- PNPase
polynucleotide phosphorylase
- PAP
poly(A) polymerase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220295997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220295997
References
- 1.Xu F, Lin-Chao S, Cohen S N. Proc Natl Acad Sci USA. 1993;90:6756–6760. doi: 10.1073/pnas.90.14.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajnsdorf E, Braun F, Haugel-Nielsen J, Regnier P. Proc Natl Acad Sci USA. 1995;92:3973–3977. doi: 10.1073/pnas.92.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Hara E B, Chekanova J A, Ingle C A, Kushner Z R, Peters E, Kushner S R. Proc Natl Acad Sci USA. 1995;92:1807–1811. doi: 10.1073/pnas.92.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn G A, Mackie G A. J Biol Chem. 1996;271:15776–15781. doi: 10.1074/jbc.271.26.15776. [DOI] [PubMed] [Google Scholar]
- 5.Mohanty B K, Kushner S R. Mol Microbiol. 1999;34:1094–1108. doi: 10.1046/j.1365-2958.1999.01673.x. [DOI] [PubMed] [Google Scholar]
- 6.Cao G-J, Pogliano J, Sarkar N. Proc Natl Acad Sci USA. 1996;93:11580–11585. doi: 10.1073/pnas.93.21.11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohanty B K, Kushner S R. Mol Microbiol. 1999;34:1109–1119. doi: 10.1046/j.1365-2958.1999.01674.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson M D, Popowski J, Cao G J, Shen P, Sarkar N. Mol Microbiol. 1998;27:23–30. doi: 10.1046/j.1365-2958.1998.00649.x. [DOI] [PubMed] [Google Scholar]
- 9.Cao G-J, Sarkar N. Biochem Biophys Res Commun. 1997;239:46–50. doi: 10.1006/bbrc.1997.7421. [DOI] [PubMed] [Google Scholar]
- 10.Sippel A E. Eur J Biochem. 1973;37:31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 11.Yehudai-Resheff S, Schuster G. Nucleic Acids Res. 2000;28:1139–1144. doi: 10.1093/nar/28.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue D, Maizels N, Weiner A M. RNA. 1996;2:895–908. [PMC free article] [PubMed] [Google Scholar]
- 13.Grunberg-Manago M. Prog Nucleic Acid Res Mol Biol. 1963;1:93–133. doi: 10.1016/s0079-6603(08)60474-2. [DOI] [PubMed] [Google Scholar]
- 14.Soreq H, Littauer U Z. J Biol Chem. 1977;252:6885–6888. [PubMed] [Google Scholar]
- 15.Kelly K O, Deutscher M P. J Biol Chem. 1992;267:17153–17158. [PubMed] [Google Scholar]
- 16.Shulman R G, Brown T R, Ugurbil K, Ogawa S, Cohen S M, den Hollander J A. Science. 1979;205:160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- 17.Donovan W P, Kushner S R. Proc Natl Acad Sci USA. 1986;83:120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn G A, Mackie G A. Prog Nucleic Acid Res Mol Biol. 1999;62:55–108. doi: 10.1016/s0079-6603(08)60505-x. [DOI] [PubMed] [Google Scholar]
- 19.Carre D S, Litvak S, Chaperville F. Biochim Biophys Acta. 1974;361:185–197. doi: 10.1016/0005-2787(74)90346-3. [DOI] [PubMed] [Google Scholar]
- 20.Williams K R, Schofield P. J Biol Chem. 1977;252:5589–5597. [PubMed] [Google Scholar]
- 21.Mohanty B K, Kushner S R. Mol Microbiol. 2000;36:982–994. doi: 10.1046/j.1365-2958.2000.01921.x. [DOI] [PubMed] [Google Scholar]
- 22.Arraiano C M, Yancey S D, Kushner S R. J Bacteriol. 1988;170:4625–4633. doi: 10.1128/jb.170.10.4625-4633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancey S D, Kushner S R. Biochimie. 1990;72:835–843. doi: 10.1016/0300-9084(90)90193-k. [DOI] [PubMed] [Google Scholar]
- 24.Jensen K F, Andersen J T, Poulsen P. J Biol Chem. 1992;267:17147–17152. [PubMed] [Google Scholar]
- 25.Cudny H, Deutscher M P. J Biol Chem. 1986;261:6450–6453. [PubMed] [Google Scholar]
- 26.Wang R F, Kushner S R. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 27.England T E, Bruce A G, Uhlenbeck O C. Methods Enzymol. 1980;65:65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- 28.Jensen K G. J Bacteriol. 1993;175:3401–3407. doi: 10.1128/jb.175.11.3401-3407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuven N B, Zhou Z, Deutscher M P. J Biol Chem. 1997;272:33255–33259. doi: 10.1074/jbc.272.52.33255. [DOI] [PubMed] [Google Scholar]
- 30.Godefroy-Colburn T, Grunberg-Manago M. In: The Enzymes. Boyer P D, editor. Vol. 7. New York: Academic; 1972. pp. 533–574. [Google Scholar]
- 31.Haugel-Nielsen J, Hajnsdorf E, Regnier P. EMBO J. 1996;15:3144–3152. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z, Deutscher M P. J Bacteriol. 1997;179:4391–4395. doi: 10.1128/jb.179.13.4391-4395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ost K A, Deutscher M P. Biochimie. 1990;72:813–818. doi: 10.1016/0300-9084(90)90190-r. [DOI] [PubMed] [Google Scholar]
- 34.Kelly K O, Reuven N B, Li Z, Deutscher M P. J Biol Chem. 1992;267:16015–16018. [PubMed] [Google Scholar]
- 35.Li Z, Deutscher M P. J Biol Chem. 1994;269:6064–6071. [PubMed] [Google Scholar]
- 36.Kushner S R. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low J K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Vol. 1. Washington, DC: Am. Soc. Microbiol. Press; 1996. pp. 849–860. [Google Scholar]
- 37.Blum E, Carpousis A J, Higgins C F. J Biol Chem. 1999;274:4009–4016. doi: 10.1074/jbc.274.7.4009. [DOI] [PubMed] [Google Scholar]