Abstract
BACKGROUND—Measles virus is implicated in the aetiology of Crohn's disease. This measles hypothesis is mainly supported by immunohistochemical findings that the measles related antigen is present in the intestine of patients with Crohn's disease. Recently we isolated this antigen from the intestine of a patient with Crohn's disease using a molecular cloning technique and produced the monoclonal antibody against it (designated 4F12). AIM—To discover whether the measles related antigen is uniquely present in Crohn's disease. SUBJECTS/METHODS—Colonic mucosa samples from 20 patients with Crohn's disease, 20 with ulcerative colitis, 11 with non-inflammatory bowel disease (IBD) colitis, and nine controls were immunohistochemically stained with the anti-measles monoclonal antibody 4F12. The numbers of positive cells, the ratio of positive cells to nucleated cells, and the staining intensity of the positive cells were compared. Furthermore, the distribution of the measles antigen in other human organs was examined. RESULTS—Both the number of positive cells and the ratio of positive cells to nucleated cells were significantly increased in Crohn's disease, ulcerative colitis, and non-IBD colitis compared with controls (p<0.05) but were similar among the three disease groups. The staining intensity of the positive cells was also similar among the three disease groups. Small numbers of positive cells were observed in the oesophagus, stomach, duodenum, jejunum, and lung. CONCLUSIONS—The presence of the measles related antigen in the colonic mucosa was not unique to Crohn's disease. These results, together with the observation that such a measles related antigen was derived from host protein, do not support the hypothesis that measles virus causes Crohn's disease. Keywords: Crohn's disease; measles virus; immunohistochemistry; ulcerative colitis; inflammatory bowel disease; molecular mimicry
Full Text
The Full Text of this article is available as a PDF (235.2 KB).
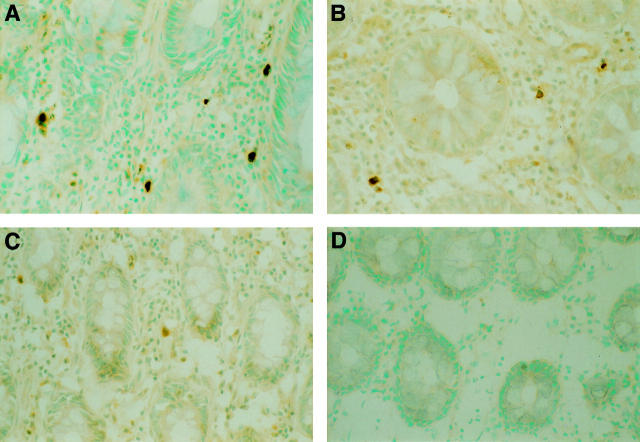
Figure 1 .
Staining intensity of positive cells: (A) strong; (B) moderate; (C) weak. No positive cells are observed in the negative control sample (D).
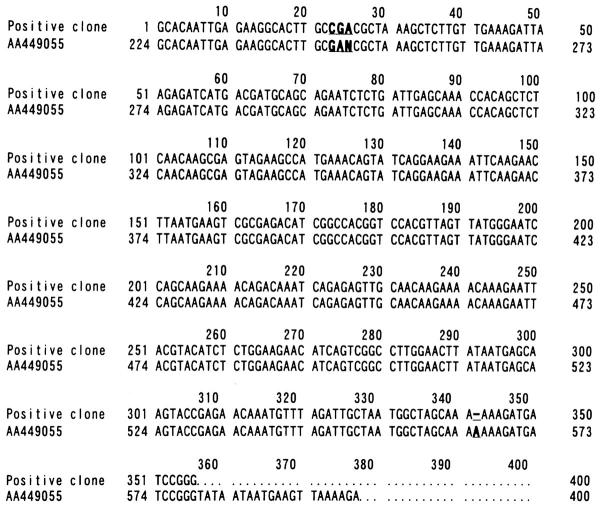
Figure 2 .
Multiple alignment of the nucleotide sequences between the positive clone and AA449055. Nucleotide sequences of non-homologous regions are shown in bold and underlined.
Figure 3 .
Deduced amino acids sequence of the positive clone. Each amino acid is shown using the single letter code.
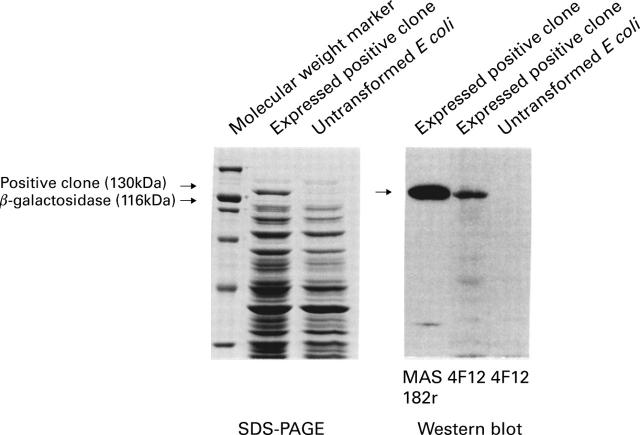
Figure 4 .
SDS/PAGE of the positive clone expressed by Escherichia coli as a fusion protein with β-galactosidase, and western blot analysis of the fusion protein (the positive clone and β-galactosidase) and anti-measles monoclonal antibodies.
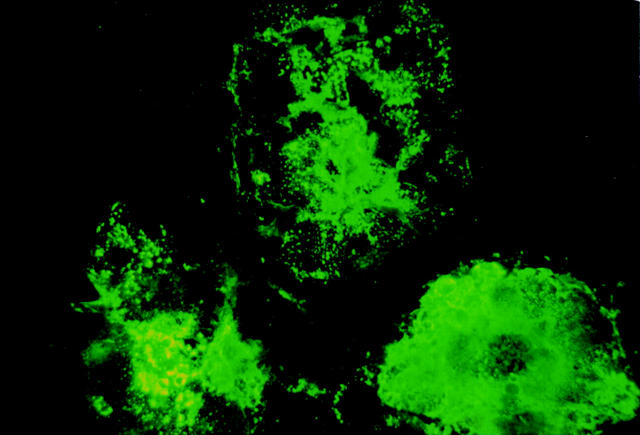
Figure 5 .
Immunofluorescence study of reaction of measles virus infected cells with 4F12. Syncytium in measles virus infected Vero cells is clearly stained with 4F12.
Figure 6 .
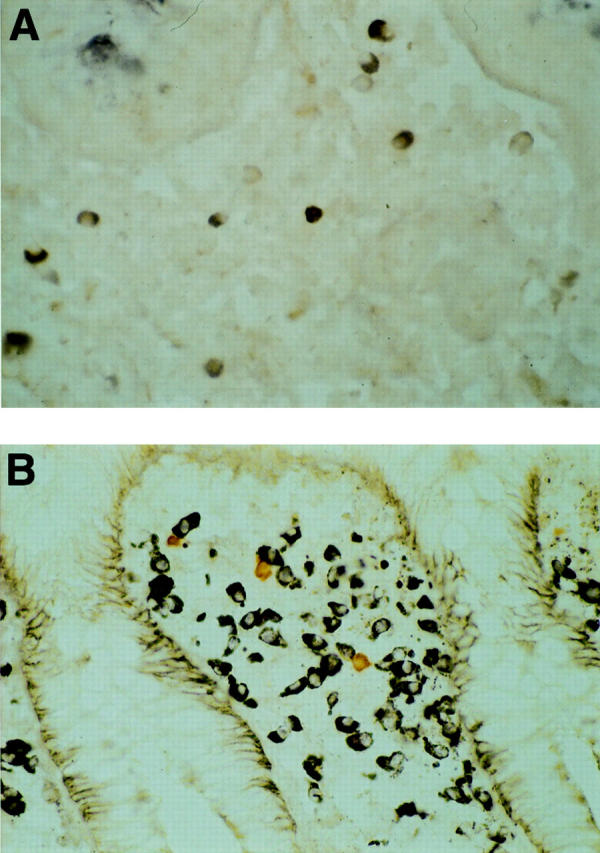
Double immunohistochemical study with MAS 182r and 4F12. Virtually all positive cells are doubly stained with MAS 182r and 4F12 (A). Doubly stained cells appear as a mixture of dark blue and brown. Cells stained singly with either 4-chloro-1-naphthol (dark blue) or diaminobenzidine (brown) are shown in (B).
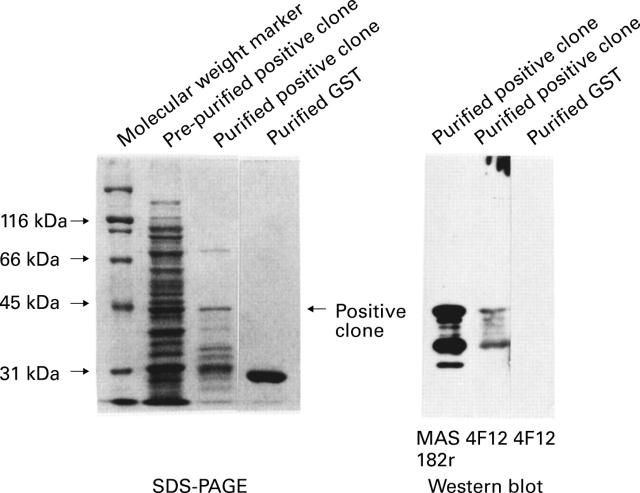
Figure 7 .
SDS/PAGE of the positive clone expressed as a fusion protein with GST, and western blot analysis of the fusion protein and anti-measles monoclonal antibodies.
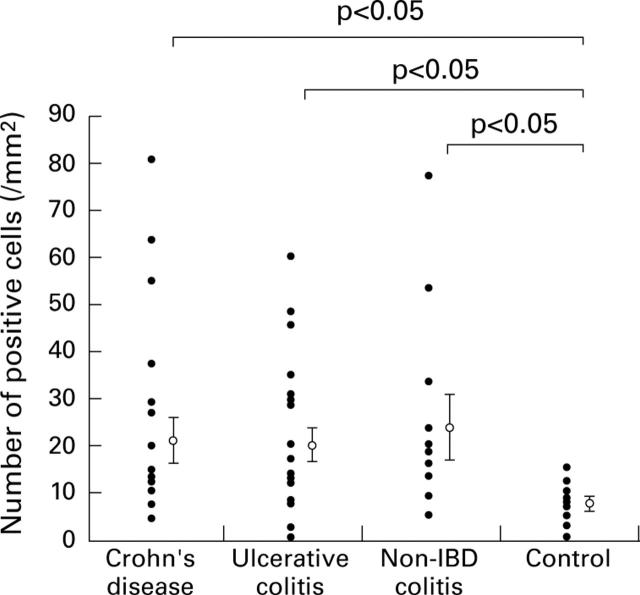
Figure 8 .
Numbers of positive cells in the colonic mucosa. The number of positive cells for each sample is shown as a closed circle. The mean and SE of the numbers of positive cells in each group is shown as an open circle and vertical bar. IBD, inflammatory bowel disease.
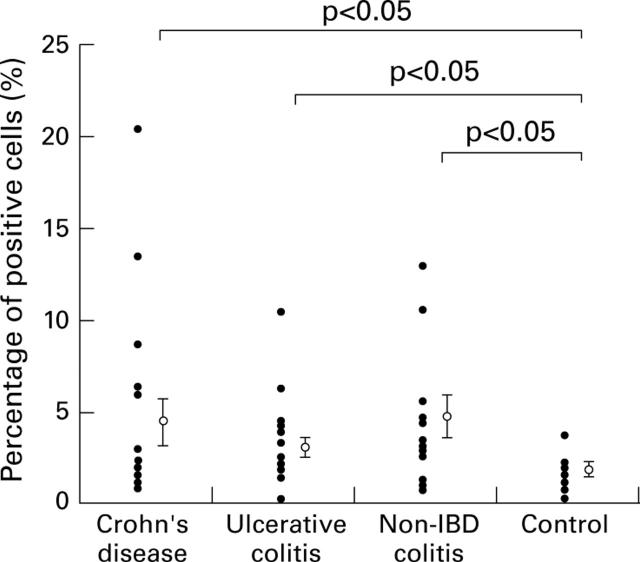
Figure 9 .
Ratio of positive cells to nucleated cells expressed as a percentage. The percentage for each sample is shown as a closed circle, and the mean and SE for each group is shown as an open circle and vertical bar.
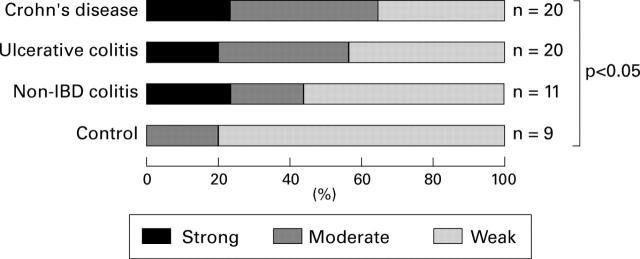
Figure 10 .
Proportions of the staining intensities in each group. The proportion staining strongly is shown as black, that staining moderately as dark grey, and that staining weakly as grey.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M. A., Armitage E., Begley J., Bentley M. L., Minor P. D., Ghosh S., Ferguson A. Absence of detectable measles virus genome sequence in inflammatory bowel disease tissues and peripheral blood lymphocytes. J Med Virol. 1998 Jul;55(3):243–249. [PubMed] [Google Scholar]
- Baxter T., Radford J. Measles vaccination as a risk factor for inflammatory bowel disease. Lancet. 1995 May 27;345(8961):1363–1364. [PubMed] [Google Scholar]
- Chadwick N., Bruce I. J., Schepelmann S., Pounder R. E., Wakefield A. J. Measles virus RNA is not detected in inflammatory bowel disease using hybrid capture and reverse transcription followed by the polymerase chain reaction. J Med Virol. 1998 Aug;55(4):305–311. [PubMed] [Google Scholar]
- Cunningham M. W., Antone S. M., Gulizia J. M., McManus B. M., Fischetti V. A., Gauntt C. J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A., Wakefield A. J., Zack M., Adami H. O. Perinatal measles infection and subsequent Crohn's disease. Lancet. 1994 Aug 20;344(8921):508–510. doi: 10.1016/s0140-6736(94)91898-8. [DOI] [PubMed] [Google Scholar]
- Farrington P., Miller E. Measles vaccination as a risk factor for inflammatory bowel disease. Lancet. 1995 May 27;345(8961):1362–1362. [PubMed] [Google Scholar]
- Feeney M., Ciegg A., Winwood P., Snook J. A case-control study of measles vaccination and inflammatory bowel disease. The East Dorset Gastroenterology Group. Lancet. 1997 Sep 13;350(9080):764–766. doi: 10.1016/s0140-6736(97)03192-9. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald Z., Sather S. D. Intraoperative cerebral infarction after desmopressin administration in infant with end-stage renal disease. Lancet. 1995 May 27;345(8961):1364–1365. doi: 10.1016/s0140-6736(95)92560-0. [DOI] [PubMed] [Google Scholar]
- Haga Y., Funakoshi O., Kuroe K., Kanazawa K., Nakajima H., Saito H., Murata Y., Munakata A., Yoshida Y. Absence of measles viral genomic sequence in intestinal tissues from Crohn's disease by nested polymerase chain reaction. Gut. 1996 Feb;38(2):211–215. doi: 10.1136/gut.38.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon-Taylor J., Ford J., Sumar N., Millar D., Doran T., Tizard M. Measles virus and Crohn's disease. Lancet. 1995 Apr 8;345(8954):922–923. doi: 10.1016/s0140-6736(95)90033-0. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Chiba M., Horie Y., Masamune O., Ohta H. Lymphoid cell subsets in colonic mucosa and HLA-DR antigens on colonic epithelia in colitis excluding ulcerative colitis and Crohn's disease. Gastroenterol Jpn. 1990 Dec;25(6):700–707. doi: 10.1007/BF02779183. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Masamune O. Measles vaccination and inflammatory bowel disease. Lancet. 1997 Dec 13;350(9093):1775–1775. doi: 10.1016/S0140-6736(05)63602-1. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Nakagomi O., Chiba M., Ueda S., Masamune O. Absence of measles virus in Crohn's disease. Lancet. 1995 Jan 21;345(8943):199–199. doi: 10.1016/s0140-6736(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Lewin J., Dhillon A. P., Sim R., Mazure G., Pounder R. E., Wakefield A. J. Persistent measles virus infection of the intestine: confirmation by immunogold electron microscopy. Gut. 1995 Apr;36(4):564–569. doi: 10.1136/gut.36.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Measles vaccination as a risk factor for inflammatory bowel disease. Lancet. 1995 May 27;345(8961):1363–1364. [PubMed] [Google Scholar]
- Metcalf J. Is measles infection associated with Crohn's disease? BMJ. 1998 Jan 17;316(7126):166–166. doi: 10.1136/bmj.316.7126.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Waight P. Measles, measles vaccination, and Crohn's disease. Second immunisation has not affected incidence in England. BMJ. 1998 Jun 6;316(7146):1745–1745. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Tanaka T., Kitamoto N., Fukuda Y., Shimoyama T. Detection of immunoreactive antigen, with a monoclonal antibody to measles virus, in tissue from a patient with Crohn's disease. J Gastroenterol. 1995 Feb;30(1):28–33. doi: 10.1007/BF01211371. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Beeler J. A. Measles vaccination and inflammatory bowel disease. Lancet. 1995 Apr 29;345(8957):1062–1063. doi: 10.1016/s0140-6736(95)90810-2. [DOI] [PubMed] [Google Scholar]
- Rugtveit J., Brandtzaeg P., Halstensen T. S., Fausa O., Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994 May;35(5):669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldenrijk C. A., Drexhage H. A., Meuwissen S. G., Pals S. T., Meijer C. J. Dendritic cells and scavenger macrophages in chronic inflammatory bowel disease. Gut. 1989 Apr;30(4):484–491. doi: 10.1136/gut.30.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. EMBO J. 1992 Jan;11(1):71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. P., Montgomery S. M., Pounder R. E., Wakefield A. J. Is measles vaccination a risk factor for inflammatory bowel disease? Lancet. 1995 Apr 29;345(8957):1071–1074. doi: 10.1016/s0140-6736(95)90816-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield A. J., Pittilo R. M., Sim R., Cosby S. L., Stephenson J. R., Dhillon A. P., Pounder R. E. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol. 1993 Apr;39(4):345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- Wakefield A. J., Sim R., Akbar A. N., Pounder R. E., Dhillon A. P. In situ immune responses in Crohn's disease: a comparison with acute and persistent measles virus infection. J Med Virol. 1997 Feb;51(2):90–100. doi: 10.1002/(sici)1096-9071(199702)51:2<90::aid-jmv2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- ter Meulen V. Measles virus and Crohn's disease: view of a medical virologist. Gut. 1998 Dec;43(6):733–734. doi: 10.1136/gut.43.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]