Full Text
The Full Text of this article is available as a PDF (166.3 KB).
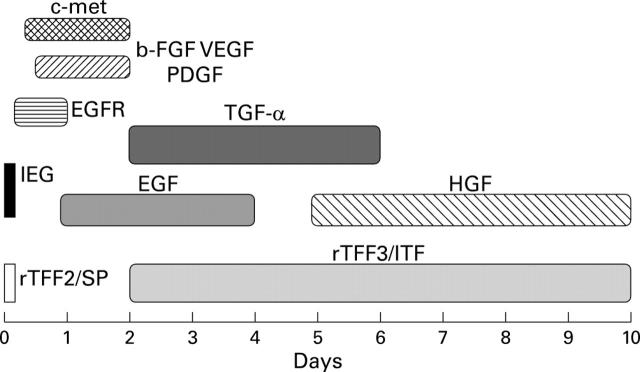
Figure 1 .
Temporal expression of genes in experimental models of ulceration in the rat. The time scale shown on the x-axis is expressed in days after mucosal injury. bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; HGF, hepatocyte growth factor; IEG, immediate early genes; PDGF, platelet derived growth factor; TGF, transforming growth factor; TFF, trefoil factors; VEGF, vascular endothelial growth factor.
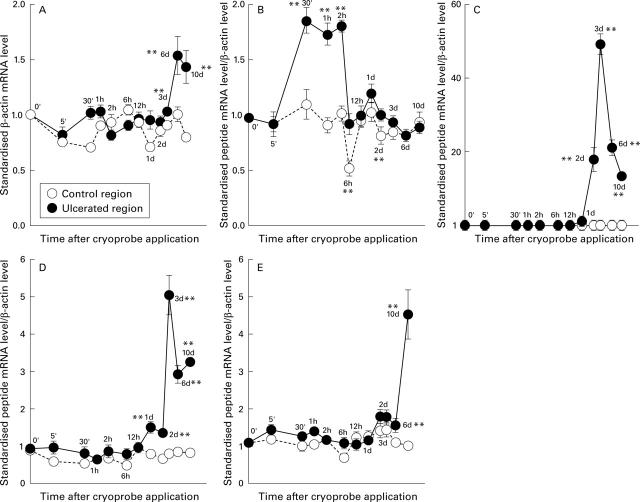
Figure 2 .
Changes in the level of mRNA after cryoprobe application in both the ulcerated region and the control region in rats. β-actin concentrations (A) were expressed relative to the corresponding region at time zero; the concentration of rTFF2/SP (B), rTFF3/ITF (C), EGF (D), and TGF-α (E) were expressed as a ratio of the specific mRNA to β-actin mRNA standardised to this ratio at time zero. **p<0.01 versus baseline values.
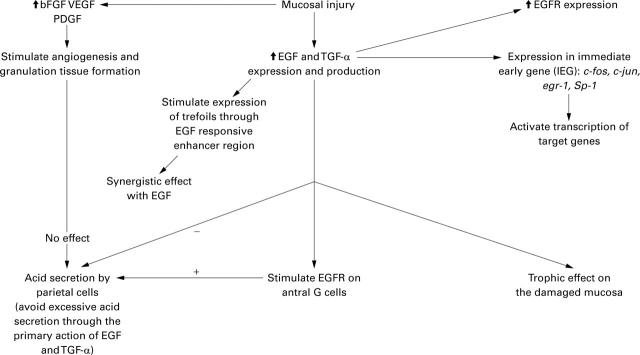
Figure 3 .
Diagram showing possible relations among epidermal growth factor (EGF), transforming growth factor (TGF) α, gastrin, trefoil peptides, basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and immediate early genes after gastric mucosal injury.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Beauchamp R. D., Russell W. E., Dubois R. N., Coffey R. J. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995 Feb;108(2):564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Polk W. H., Moses H. L., Coffey R. J. Production of transforming growth factor-alpha by normal rat small intestine. Am J Physiol. 1991 Dec;261(6 Pt 1):C994–1000. doi: 10.1152/ajpcell.1991.261.6.C994. [DOI] [PubMed] [Google Scholar]
- Barros E. J., Santos O. F., Matsumoto K., Nakamura T., Nigam S. K. Differential tubulogenic and branching morphogenetic activities of growth factors: implications for epithelial tissue development. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4412–4416. doi: 10.1073/pnas.92.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartlidge S. A., Elder J. B. Transforming growth factor alpha and epidermal growth factor levels in normal human gastrointestinal mucosa. Br J Cancer. 1989 Nov;60(5):657–660. doi: 10.1038/bjc.1989.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinery R., Playford R. J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995 Apr;88(4):401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Yeomans N. D., Giraud A. S. Temporal expression of trefoil peptides in the TGF-alpha knockout mouse after gastric ulceration. Am J Physiol. 1997 Jun;272(6 Pt 1):G1540–G1549. doi: 10.1152/ajpgi.1997.272.6.G1540. [DOI] [PubMed] [Google Scholar]
- Dignass A. U., Lynch-Devaney K., Podolsky D. K. Hepatocyte growth factor/scatter factor modulates intestinal epithelial cell proliferation and migration. Biochem Biophys Res Commun. 1994 Jul 29;202(2):701–709. doi: 10.1006/bbrc.1994.1987. [DOI] [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gak E., Taylor W. G., Chan A. M., Rubin J. S. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992 Oct 12;311(1):17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- Gohda E., Tsubouchi H., Nakayama H., Hirono S., Sakiyama O., Takahashi K., Miyazaki H., Hashimoto S., Daikuhara Y. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest. 1988 Feb;81(2):414–419. doi: 10.1172/JCI113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Epidermal growth factor (EGF). Baillieres Clin Gastroenterol. 1996 Mar;10(1):33–47. doi: 10.1016/s0950-3528(96)90038-x. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Epidermal growth factor and transforming growth factor-alpha actions on the gut. Eur J Gastroenterol Hepatol. 1995 Oct;7(10):928–932. doi: 10.1097/00042737-199510000-00004. [DOI] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990 Jul;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmrath M. A., Shin C. E., Erwin C. R., Warner B. W. Epidermal growth factor upregulates the expression of its own intestinal receptor after small bowel resection. J Pediatr Surg. 1998 Feb;33(2):229–234. doi: 10.1016/s0022-3468(98)90437-7. [DOI] [PubMed] [Google Scholar]
- Hull M. A., Brough J. L., Powe D. G., Carter G. I., Jenkins D., Hawkey C. J. Expression of basic fibroblast growth factor in intact and ulcerated human gastric mucosa. Gut. 1998 Oct;43(4):525–536. doi: 10.1136/gut.43.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull M. A., Cullen D. J., Hudson N., Hawkey C. J. Basic fibroblast growth factor treatment for non-steroidal anti-inflammatory drug associated gastric ulceration. Gut. 1995 Nov;37(5):610–612. doi: 10.1136/gut.37.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Hayashi N., Sasaki Y., Morita Y., Kawano S., Fusamoto H., Sato N., Tohyama M., Kamada T. Sequential protooncogene expression during regeneration in rat stomach. Gastroenterology. 1990 Jun;98(6):1525–1531. doi: 10.1016/0016-5085(90)91085-k. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Kishi K., Asahara M., Matasushima Y., Wang H. Y., Miyazawa K., Kitamura N., Chiba T. Production and activation of hepatocyte growth factor during the healing of rat gastric ulcers. Digestion. 1997;58(3):225–231. doi: 10.1159/000201448. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Nakata H., Hassan S., Asahara M., Kawanami C., Matsushima Y., Naribayashi-Inomoto Y., Ping C. Y., Min D., Nakamura A. Gene expression of keratinocyte and hepatocyte growth factors during the healing of rat gastric mucosal lesions. Gastroenterology. 1995 Oct;109(4):1068–1077. doi: 10.1016/0016-5085(95)90564-2. [DOI] [PubMed] [Google Scholar]
- Konturek P. C., Brzozowski T., Konturek S. J., Ernst H., Drozdowicz D., Pajdo R., Hahn E. G. Expression of epidermal growth factor and transforming growth factor alpha during ulcer healing. Time sequence study. Scand J Gastroenterol. 1997 Jan;32(1):6–15. doi: 10.3109/00365529709025056. [DOI] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994 Nov;19(11):459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Lokker N. A., Mark M. R., Luis E. A., Bennett G. L., Robbins K. A., Baker J. B., Godowski P. J. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992 Jul;11(7):2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A. P., Fligiel S. E., Jaszewski R., Tureaud J., Dutta S., Chelluderai B. Inhibition of gastric mucosal regeneration by tyrphostin: evaluation of the role of epidermal growth factor receptor tyrosine kinase. J Lab Clin Med. 1996 Aug;128(2):173–180. doi: 10.1016/s0022-2143(96)90009-8. [DOI] [PubMed] [Google Scholar]
- Marchbank T., Freeman T. C., Playford R. J. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59(3):167–174. doi: 10.1159/000007485. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Monroe J. G. Role of primary response genes in generating cellular responses to growth factors. FASEB J. 1992 Jun;6(9):2707–2715. doi: 10.1096/fasebj.6.9.1612295. [DOI] [PubMed] [Google Scholar]
- Merchant J. L., Demediuk B., Brand S. J. A GC-rich element confers epidermal growth factor responsiveness to transcription from the gastrin promoter. Mol Cell Biol. 1991 May;11(5):2686–2696. doi: 10.1128/mcb.11.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen P. J., Berger J. E., Meneses J., Phung Y., Pedersen R. A., Werb Z., Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995 Jul 27;376(6538):337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Shimomura T., Naka D., Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem. 1994 Mar 25;269(12):8966–8970. [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Naka D., Ishii T., Yoshiyama Y., Miyazawa K., Hara H., Hishida T., Kidamura N. Activation of hepatocyte growth factor by proteolytic conversion of a single chain form to a heterodimer. J Biol Chem. 1992 Oct 5;267(28):20114–20119. [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B. L., Sha'afi R. I., Hajjar J. J. Transforming growth factor-alpha and epidermal growth factor activate mitogen-activated protein kinase and its substrates in intestinal epithelial cells. Proc Soc Exp Biol Med. 1995 Nov;210(2):162–170. doi: 10.3181/00379727-210-43936. [DOI] [PubMed] [Google Scholar]
- Olsen P. S., Poulsen S. S., Therkelsen K., Nexø E. Effect of sialoadenectomy and synthetic human urogastrone on healing of chronic gastric ulcers in rats. Gut. 1986 Dec;27(12):1443–1449. doi: 10.1136/gut.27.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Batten J. J., Freeman T. C., Beardshall K., Vesey D. A., Fenn G. C., Baron J. H., Calam J. Gastric output of pancreatic secretory trypsin inhibitor is increased by misoprostol. Gut. 1991 Nov;32(11):1396–1400. doi: 10.1136/gut.32.11.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Hanby A. M., Gschmeissner S., Peiffer L. P., Wright N. A., McGarrity T. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996 Aug;39(2):262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Mandir N., Higham A., Meeran K., Ghatei M. A., Bloom S. R., Goodlad R. A. Effects of keratinocyte growth factor (KGF) on gut growth and repair. J Pathol. 1998 Mar;184(3):316–322. doi: 10.1002/(SICI)1096-9896(199803)184:3<316::AID-PATH3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Playford R. J. Peptides and gastrointestinal mucosal integrity. Gut. 1995 Nov;37(5):595–597. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk W. H., Jr, Dempsey P. J., Russell W. E., Brown P. I., Beauchamp R. D., Barnard J. A., Coffey R. J., Jr Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology. 1992 May;102(5):1467–1474. doi: 10.1016/0016-5085(92)91703-7. [DOI] [PubMed] [Google Scholar]
- Poulsom R. Trefoil peptides. Baillieres Clin Gastroenterol. 1996 Mar;10(1):113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Relan N. K., Fligiel S. E., Dutta S., Tureaud J., Chauhan D. P., Majumdar A. P. Induction of EGF-receptor tyrosine kinase during early reparative phase of gastric mucosa and effects of aging. Lab Invest. 1995 Nov;73(5):717–726. [PubMed] [Google Scholar]
- Rhodes J. A., Tam J. P., Finke U., Saunders M., Bernanke J., Silen W., Murphy R. A. Transforming growth factor alpha inhibits secretion of gastric acid. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3844–3846. doi: 10.1073/pnas.83.11.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. A. Immediate-early genes, neuronal plasticity, and memory. Biochem Cell Biol. 1992 Sep;70(9):729–737. doi: 10.1139/o92-112. [DOI] [PubMed] [Google Scholar]
- Romano M., Polk W. H., Awad J. A., Arteaga C. L., Nanney L. B., Wargovich M. J., Kraus E. R., Boland C. R., Coffey R. J. Transforming growth factor alpha protection against drug-induced injury to the rat gastric mucosa in vivo. J Clin Invest. 1992 Dec;90(6):2409–2421. doi: 10.1172/JCI116132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands B. E., Podolsky D. K. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- Satoh H., Shino A., Sato F., Asano S., Murakami I., Inatomi N., Nagaya H., Kato K., Szabo S., Folkman J. Role of endogenous basic fibroblast growth factor in the healing of gastric ulcers in rats. Jpn J Pharmacol. 1997 Jan;73(1):59–71. doi: 10.1254/jjp.73.59. [DOI] [PubMed] [Google Scholar]
- Schmassmann A., Stettler C., Poulsom R., Tarasova N., Hirschi C., Flogerzi B., Matsumoto K., Nakamura T., Halter F. Roles of hepatocyte growth factor and its receptor Met during gastric ulcer healing in rats. Gastroenterology. 1997 Dec;113(6):1858–1872. doi: 10.1016/s0016-5085(97)70005-2. [DOI] [PubMed] [Google Scholar]
- Szabo S., Folkman J., Vattay P., Morales R. E., Pinkus G. S., Kato K. Accelerated healing of duodenal ulcers by oral administration of a mutein of basic fibroblast growth factor in rats. Gastroenterology. 1994 Apr;106(4):1106–1111. doi: 10.1016/0016-5085(94)90773-0. [DOI] [PubMed] [Google Scholar]
- Szabo S., Sandor Z. Basic fibroblast growth factor and PDGF in GI diseases. Baillieres Clin Gastroenterol. 1996 Mar;10(1):97–112. doi: 10.1016/s0950-3528(96)90042-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ota S., Shimada T., Hamada E., Kawabe T., Okudaira T., Matsumura M., Kaneko N., Terano A., Nakamura T. Hepatocyte growth factor is the most potent endogenous stimulant of rabbit gastric epithelial cell proliferation and migration in primary culture. J Clin Invest. 1995 May;95(5):1994–2003. doi: 10.1172/JCI117884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A., Stachura J., Durbin T., Sarfeh I. J., Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992 Feb;102(2):695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Stachura J., Krause W. J., Douglass T. G., Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13 (Suppl 1):S42–S47. doi: 10.1097/00004836-199112001-00007. [DOI] [PubMed] [Google Scholar]
- Thomas D. M., Nasim M. M., Gullick W. J., Alison M. R. Immunoreactivity of transforming growth factor alpha in the normal adult gastrointestinal tract. Gut. 1992 May;33(5):628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarfaty I., Resau J. H., Rulong S., Keydar I., Faletto D. L., Vande Woude G. F. The met proto-oncogene receptor and lumen formation. Science. 1992 Aug 28;257(5074):1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kawano S., Tsujii M., Fusamoto H., Kamada T. Roles of hepatocyte growth factor and its receptor in gastric mucosa. A cell biological and molecular biological study. Dig Dis Sci. 1995 May;40(5):1132–1139. doi: 10.1007/BF02064211. [DOI] [PubMed] [Google Scholar]
- Tsujii M., Kawano S., Tsuji S., Ito T., Hayashi N., Horimoto M., Mita E., Nagano K., Masuda E., Hayashi N. Increased expression of c-met messenger RNA following acute gastric injury in rats. Biochem Biophys Res Commun. 1994 Apr 15;200(1):536–541. doi: 10.1006/bbrc.1994.1481. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Johnson L. R. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol. 1994 May;266(5 Pt 1):G878–G886. doi: 10.1152/ajpgi.1994.266.5.G878. [DOI] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Wright N. A., Hoffmann W., Otto W. R., Rio M. C., Thim L. Rolling in the clover: trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997 May 19;408(2):121–123. doi: 10.1016/s0014-5793(97)00424-9. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Kondo S., Murayama Y., Nishibayashi H., Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996 Dec;39(6):787–794. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura K., Ota S., Terano A., Takahashi M., Hata Y., Kawabe T., Mutoh H., Hiraishi H., Nakata R., Okano K. Growth regulation of rabbit gastric epithelial cells and protooncogene expression. Dig Dis Sci. 1994 Jul;39(7):1454–1463. doi: 10.1007/BF02088048. [DOI] [PubMed] [Google Scholar]
- Zeeh J. M., Procaccino F., Hoffmann P., Aukerman S. L., McRoberts J. A., Soltani S., Pierce G. F., Lakshmanan J., Lacey D., Eysselein V. E. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996 Apr;110(4):1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]