Abstract
BACKGROUND—Reactive oxygen species are implicated in the aetiology of a range of human diseases and there is increasing interest in their role in the development of cancer. AIM—To develop a suitable method for the detection of reactive oxygen species produced by the faecal matrix. METHODS—A refined high performance liquid chromatography system for the detection of reactive oxygen species is described. RESULTS—The method allows baseline separation of the products of hydroxyl radical attack on salicylic acid in the hypoxanthine/xanthine oxidase system, namely 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid, and catechol. The increased efficiency and precision of the method has allowed a detailed evaluation of the dynamics of reactive oxygen species generation in the faecal matrix. The data show that the faecal matrix is capable of generating reactive oxygen species in abundance. This ability cannot be attributed to the bacteria present, but rather to a soluble component within the matrix. As yet, the nature of this soluble factor is not entirely clear but is likely to be a reducing agent. CONCLUSIONS—The soluble nature of the promoting factor renders it amenable to absorption, and circumstances may exist in which either it comes into contact with either free or chelated iron in the colonocyte, leading to direct attack on cellular DNA, or else it initiates lipid peroxidation processes whereby membrane polyunsaturated fatty acids are attacked by reactive oxygen species propagating chain reactions leading to the generation of promutagenic lesions such as etheno based DNA adducts. Keywords: colorectal cancer; faecal matrix; hypoxanthine; phytic acid; reactive oxygen species; xanthine oxidase
Full Text
The Full Text of this article is available as a PDF (200.3 KB).
Figure 1 .
Reaction sequence for the analysis of reactive oxygen species in the hypoxanthine/xanthine oxidase and faecal systems.
Figure 2 .
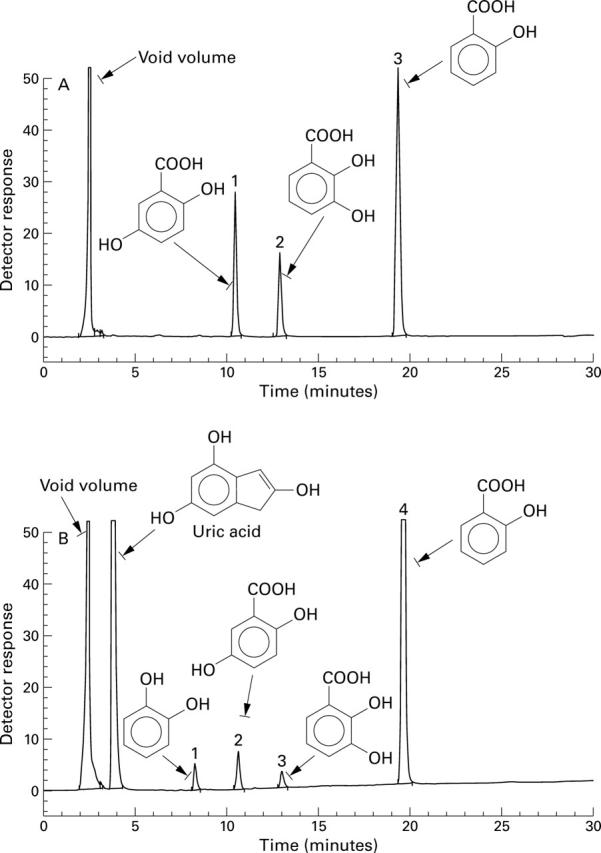
(A) High performance liquid chromatogram of salicylic acid (3) and its hydroxylated products 2,5-dihydroxybenzoic acid (1) and 2,3-dihydroxybenzoic acid (2) with diode array detector set at 325 nm. (B) High performance liquid chromatogram of salicylic acid (4) and its decarboxylated product, catechol (1), and the hydroxylated products, 2,5-dihydroxybenzoic acid (2) and 2,3-dihydroxybenzoic acid (3), with the diode array detector set at 278 nm and 325 nm.
Figure 3 .
Comparison of the phosphate and Tris buffer systems for the detection of reactive oxygen species attack on salicylic acid.
Figure 4 .
Effect of various scavengers on the detection of reactive oxygen species in the phosphate buffer system. DMSO, dimethyl sulphoxide.
Figure 5 .
Inhibition of the generation of reactive oxygen species by phytic acid in an EDTA deplete phosphate buffer system.
Figure 6 .
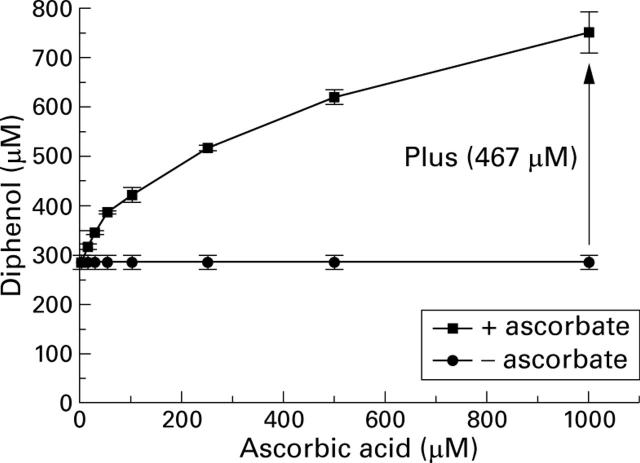
Promotion of the generation of reactive oxygen species by ascorbic acid in the complete phosphate buffer system.
Figure 7 .
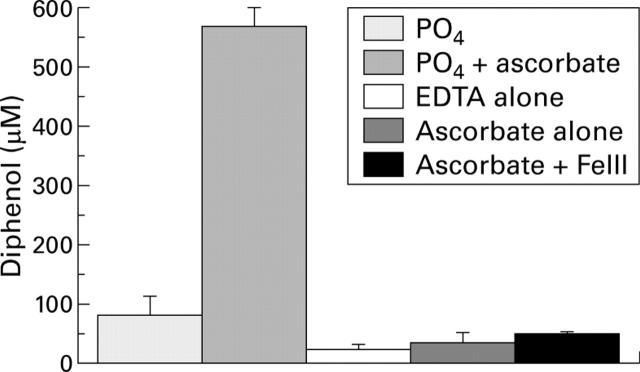
Effect of ascorbic acid (500 µM) on the generation of reactive oxygen species in variations of the phosphate buffer system.
Figure 8 .
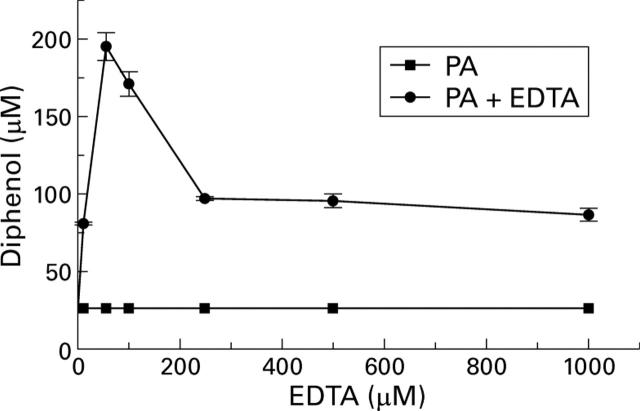
Effect of EDTA on phytic acid (PA; 500 µM) inhibition of generation of reactive oxygen species in the hypoxanthine/xanthine oxidase assay.
Figure 9 .
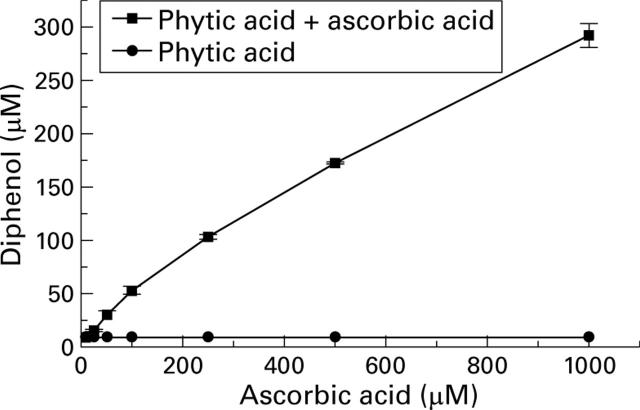
Effect of ascorbic acid on phytic acid (500 µM) inhibition of reactive oxygen species generation in the hypoxanthine/xanthine oxidase assay.
Figure 10 .
Generation of reactive oxygen species in faecal samples (n = 4) from adenoma patients under various conditions. HX, hypoxanthine.
Figure 11 .
Effect of various scavengers on the detection of reactive oxygen species in faecal samples (n = 4). DMSO, dimethyl sulphoxide.
Figure 12 .
Effect of EDTA (500 µM) and ascorbic acid (500 µM) on the generation of reactive oxygen species in faecal samples.
Figure 13 .
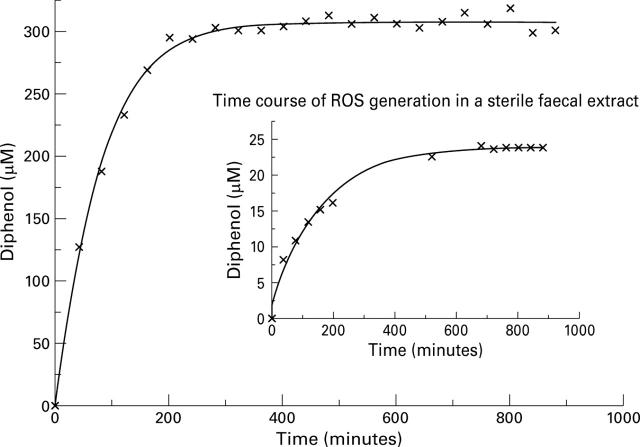
Time course of the generation of reactive oxygen species (ROS) in the complete phosphate hypoxanthine/xanthine oxidase system compared with that of faecal samples (inset). Note: The experiments were conducted at room temperature (25°C) and the results are therefore not directly comparable with the data in table 1 (conducted at 37°C).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbs C. F. Free radicals and the etiology of colon cancer. Free Radic Biol Med. 1990;8(2):191–200. doi: 10.1016/0891-5849(90)90091-v. [DOI] [PubMed] [Google Scholar]
- Babbs C. F., Gale M. J. Colorimetric assay for methanesulfinic acid in biological samples. Anal Biochem. 1987 May 15;163(1):67–73. doi: 10.1016/0003-2697(87)90093-5. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Nair J., Velic I. Etheno-DNA base adducts as tools in human cancer aetiology and chemoprevention. Eur J Cancer Prev. 1997 Dec;6(6):529–534. doi: 10.1097/00008469-199712000-00007. [DOI] [PubMed] [Google Scholar]
- Consensus meeting on cereals, fibre and colorectal and breast cancers. ECP consensus panel on cereals and cancer. Eur J Cancer Prev. 1997 Dec;6(6):512–514. doi: 10.1097/00008469-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Graf E., Eaton J. W. Antioxidant functions of phytic acid. Free Radic Biol Med. 1990;8(1):61–69. doi: 10.1016/0891-5849(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Graf E., Eaton J. W. Dietary suppression of colonic cancer. Fiber or phytate? Cancer. 1985 Aug 15;56(4):717–718. doi: 10.1002/1097-0142(19850815)56:4<717::aid-cncr2820560402>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986 Jul 15;237(2):499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989 Dec;70(6):737–757. [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A., Olyaee M., Sontag S., Mobarhan S. Increased levels of luminol-enhanced chemiluminescence by rectal mucosa of patients with colonic neoplasia: a possible marker for colonic neoplasia. Nutr Cancer. 1993;19(2):201–206. doi: 10.1080/01635589309514250. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Sedghi S., Kanofsky J., List T., Robinson C., Ibrahim C., Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992 Jul;103(1):177–185. doi: 10.1016/0016-5085(92)91111-g. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Cohen G., Cederbaum A. I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry. 1981 Oct 13;20(21):6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Nair J., Vaca C. E., Velic I., Mutanen M., Valsta L. M., Bartsch H. High dietary omega-6 polyunsaturated fatty acids drastically increase the formation of etheno-DNA base adducts in white blood cells of female subjects. Cancer Epidemiol Biomarkers Prev. 1997 Aug;6(8):597–601. [PubMed] [Google Scholar]
- Nair U. J., Obe G., Friesen M., Goldberg M. T., Bartsch H. Role of lime in the generation of reactive oxygen species from betel-quid ingredients. Environ Health Perspect. 1992 Nov;98:203–205. doi: 10.1289/ehp.9298203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Yoo S. J., Tanure J. C., Andrianopoulos G., Misumi A. The effect of iron on experimental colorectal carcinogenesis. Anticancer Res. 1989 Nov-Dec;9(6):1477–1482. [PubMed] [Google Scholar]
- Owen R. W., Spiegelhalder B., Bartsch H. Phytate, reactive oxygen species and colorectal cancer. Eur J Cancer Prev. 1998 May;7 (Suppl 2):S41–S54. doi: 10.1097/00008469-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Owen R. W., Weisgerber U. M., Spiegelhalder B., Bartsch H. Faecal phytic acid and its relation to other putative markers of risk for colorectal cancer. Gut. 1996 Apr;38(4):591–597. doi: 10.1136/gut.38.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. W., Wimonwatwatee T., Spiegelhalder B., Bartsch H. A high performance liquid chromatography system for quantification of hydroxyl radical formation by determination of dihydroxy benzoic acids. Eur J Cancer Prev. 1996 Aug;5(4):233–240. doi: 10.1097/00008469-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Richmond R., Halliwell B., Chauhan J., Darbre A. Superoxide-dependent formation of hydroxyl radicals: detection of hydroxyl radicals by the hydroxylation of aromatic compounds. Anal Biochem. 1981 Dec;118(2):328–335. doi: 10.1016/0003-2697(81)90590-x. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. M., Elsayed A. M., Ullah A. Suppression of large intestinal cancer in F344 rats by inositol hexaphosphate. Carcinogenesis. 1988 Apr;9(4):577–580. doi: 10.1093/carcin/9.4.577. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. M., Ullah A. Inositol hexaphosphate inhibits large intestinal cancer in F344 rats 5 months after induction by azoxymethane. Carcinogenesis. 1989 Mar;10(3):625–626. doi: 10.1093/carcin/10.3.625. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Bumann D., Baretton G., Younes M. Dietary iron enhances the tumor rate in dimethylhydrazine-induced colon carcinogenesis in mice. Cancer Lett. 1988 Aug 30;41(3):251–256. doi: 10.1016/0304-3835(88)90285-6. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Bumann D., Trepkau H. D., Schadwinkel B., Baretton G. Influence of dietary iron overload on cell proliferation and intestinal tumorigenesis in mice. Cancer Lett. 1992 Aug 31;65(3):245–249. doi: 10.1016/0304-3835(92)90239-r. [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Anders F. The involvement of reactive oxygen species in oral cancers of betel quid/tobacco chewers. Mutat Res. 1989 Sep;214(1):47–61. doi: 10.1016/0027-5107(89)90197-8. [DOI] [PubMed] [Google Scholar]
- Thompson L. U., Zhang L. Phytic acid and minerals: effect on early markers of risk for mammary and colon carcinogenesis. Carcinogenesis. 1991 Nov;12(11):2041–2045. doi: 10.1093/carcin/12.11.2041. [DOI] [PubMed] [Google Scholar]
- Ullah A., Shamsuddin A. M. Dose-dependent inhibition of large intestinal cancer by inositol hexaphosphate in F344 rats. Carcinogenesis. 1990 Dec;11(12):2219–2222. doi: 10.1093/carcin/11.12.2219. [DOI] [PubMed] [Google Scholar]
- Weisgerber U. M., Boeing H., Owen R. W., Waldherr R., Raedsch R., Wahrendorf J. Effect of longterm placebo controlled calcium supplementation on sigmoidal cell proliferation in patients with sporadic adenomatous polyps. Gut. 1996 Mar;38(3):396–402. doi: 10.1136/gut.38.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]