Abstract
BACKGROUND—Increased nitric oxide (NO) synthase activity and enhanced apoptosis are features of gastric mucosa infected with Helicobacter pylori and a causative relation has been suggested. However, although NO can promote apoptosis, its actions vary with cell type. AIMS—To determine whether exogenous NO, derived from an NO donor, might promote or counteract apoptosis in gastric mucous epithelial cells. METHODS—Primary cultures of guinea pig gastric mucosal cells were exposed to the NO donor S-nitroso-N-acetyl-penicillamine (SNAP) for 24 hours. Apoptosis was detected from nuclear staining with Hoechst 33258, in situ nick end labelling of DNA, and the presence of DNA "ladders" in cell extracts. Cyclic GMP content and caspase activity were determined by immunoassay and fluorimetric assay respectively. RESULTS—SNAP 1 mM did not alter the small proportion of cells on the culture plate (3-6%) which exhibited features of apoptosis. However, SNAP produced an inhibition of apoptosis, and of caspase 3 like activity, when enhanced by 25 µM N-hexanoyl-D-sphingosine (C6-ceramide), or by detachment of cells from the culture plate. The guanylate cyclase inhibitor, 1H-1, 2, 4-oxadiazole-4, 3-a-quinoxaline-1-one (ODQ), prevented the stimulation of cyclic GMP by SNAP, but not the anti- apoptotic effects of the NO donor. The cyclic GMP analogues 8-bromo-cyclic GMP and 8-(4-chlorophenylthio) guanosine-3',5'- cyclic monophosphate did not significantly inhibit apoptosis in the mucosal cells. CONCLUSIONS—Exogenous NO inhibited apoptosis in guinea pig gastric mucous cells by a mechanism which did not involve elevation of cyclic GMP. NO, if produced from NO synthase during infection with H pylori, may therefore counter the proapoptotic effects of this pathogen. Keywords: nitric oxide; gastric mucosa; stomach; apoptosis
Full Text
The Full Text of this article is available as a PDF (174.5 KB).
Figure 1 .
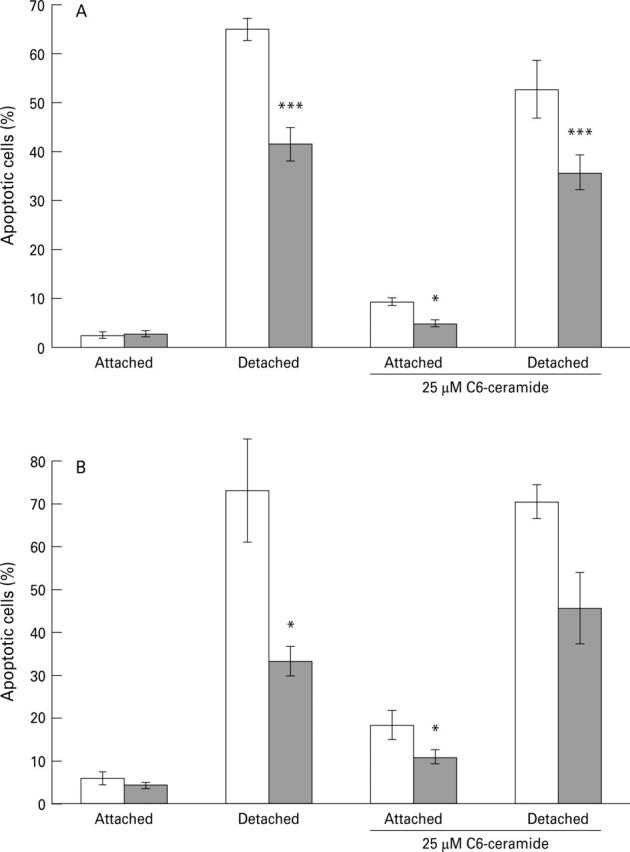
Effect of 1 mM S-nitroso-N-acetyl-penicillamine (SNAP; shaded bars) on apoptosis in guinea pig gastric mucosal cells in the presence and absence of 25 µM C6-ceramide. Apoptosis was detected by staining of nuclei with Hoechst 33258 in A and by nick end labelling in B, with data presented as mean (SEM) of six and four separate cell preparations, respectively. Data were analysed by analysis of variance followed by a Newman-Keuls multiple comparison test. *p<0.05, ***p<0.001 for the effect of SNAP in comparison with the same treatment without SNAP.
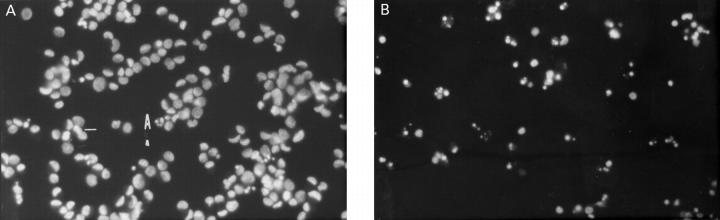
Figure 2 .
Nuclear staining with Hoechst 33258. (A) Cells treated with 1 mM S-nitroso-N-acetyl-penicillamine that were attached to the culture plate. One of the few apoptotic cells is labelled A. (B) Cells that had detached spontaneously under control conditions.
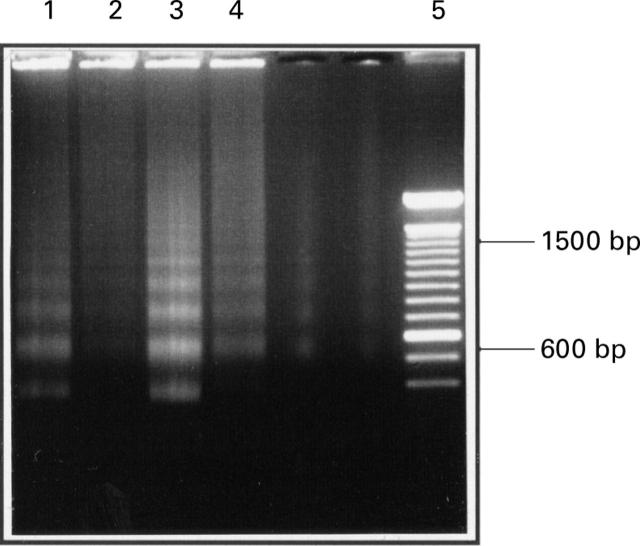
Figure 3 .
Fragmentation pattern of low molecular weight DNA as revealed by electrophoresis on agarose. Cells were treated with 25 µM C6-ceramide in the presence (lanes 2 and 4) and absence (lanes 1 and 3) of 1 mM S-nitroso- N-acetyl-penicillamine. Extracts from cells attached to the culture plate are in lanes 1 and 2 and cells that had detached are in lanes 3 and 4. Markers are in lane 5.
Figure 4 .
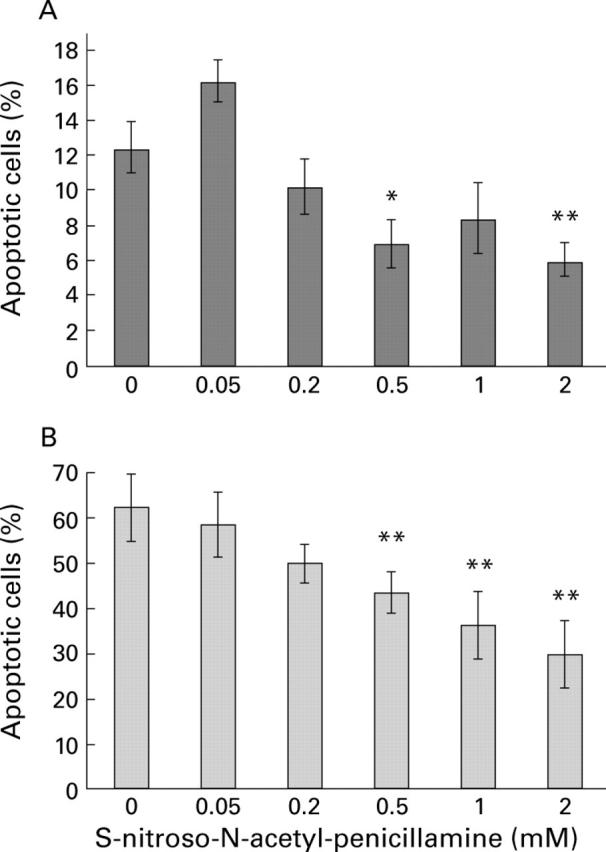
Effect of the concentration of S-nitroso-N-acetyl-penicillamine (SNAP) on apoptosis in cells attached (A) and detached (B) that had been treated with 25 µM C6-ceramide. Apoptosis was quantified by staining of nuclei with Hoechst dye. Data are the mean (SEM) of four separate cultures and have been analysed by analysis of variance followed by Dunnett's test. *p<0.05, **p<0.01 for comparison with the result obtained in the absence of SNAP.
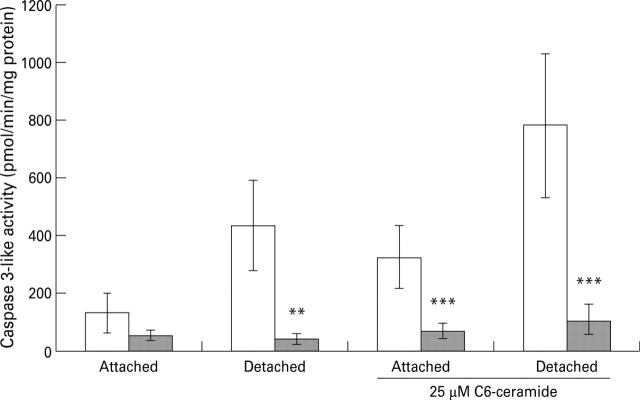
Figure 5 .
Effect of 1 mM S-nitroso-N-acetyl-penicillamine (SNAP; shaded bars) on caspase 3 like activity in extracts of guinea pig gastric mucosal cells incubated for 24 hours in the presence and absence of 25 µM C6-ceramide. Data are presented as mean (SEM) of five separate cell preparations. Data were analysed by two factor analysis of variance, to remove the effect of variation in caspase activity between experiments, followed by a Newman-Keuls multiple comparison test. **p<0.01, ***p<0.001 for the effect of SNAP in comparison with the same treatment without SNAP.
Figure 6 .
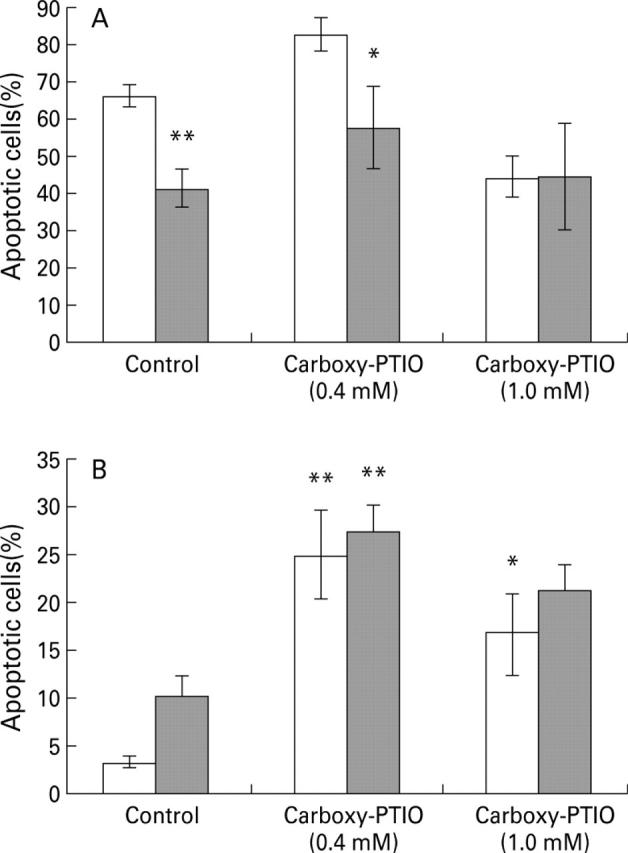
Effect of 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO) on apoptosis (Hoechst staining). (A) Data are mean (SEM) for cells which had detached from the plate in three separate experiments with the effect of 1 mM SNAP (shaded bars) determined by paired t test; *p<0.05, **p<0.01. (B) Data are for attached cells in the presence (shaded bars) and absence (clear bars) of 25 µM C6-ceramide and are mean (SEM) of four separate cell preparations; *p<0.05, **p<0.01 for the difference from the appropriate control by a Newman-Keuls test.
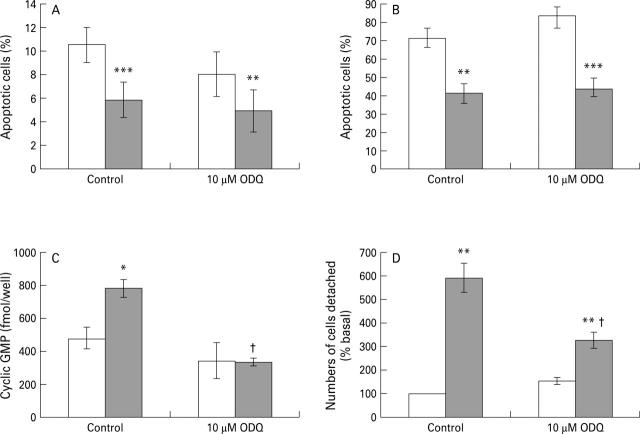
Figure 7 .
Effect of ODQ (1H-1,2,4-oxadiazole-4,3-a-quinoxaline-1-one) on the action of 1 mM S-nitroso-N-acetyl- penicillamine (SNAP, shaded bars) on apoptosis (Hoechst 33258 staining), cyclic GMP content, and on detachment of cells from the culture plate. The attached cells in panels A and C had been treated with 25 µM C6-ceramide to induce apoptosis. Results in B are for detached cells. Data in A, B, C, and D are mean (SEM) of five, four, three, and three cell preparations, respectively, and were subjected to analysis of variance followed by a Newman-Keuls multiple comparison test. *p<0.05, **p<0.01,***p<0.001 for the effect of SNAP; †p<0.05 for the effect of ODQ in the presence of SNAP.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. F., Tepperman B. L., Hanson P. J., Whittle B. J. Lipopolysaccharide induces Ca(2+)-independent nitric oxide synthase activity in rat gastric mucosal cells. Eur J Pharmacol. 1994 Nov 1;292(1):111–114. doi: 10.1016/0926-6917(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Brown J. F., Tepperman B. L., Hanson P. J., Whittle B. J., Moncada S. Differential distribution of nitric oxide synthase between cell fractions isolated from the rat gastric mucosa. Biochem Biophys Res Commun. 1992 Apr 30;184(2):680–685. doi: 10.1016/0006-291x(92)90643-y. [DOI] [PubMed] [Google Scholar]
- Brüne B., von Knethen A., Sandau K. B. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998 Jun 26;351(3):261–272. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- Byrne C. R., Hanson P. J. Induction of heat shock protein 72 by a nitric oxide donor in guinea-pig gastric mucosal cells. Eur J Pharmacol. 1998 Jul 17;353(1):117–122. doi: 10.1016/s0014-2999(98)00388-4. [DOI] [PubMed] [Google Scholar]
- Chen G., Sordillo E. M., Ramey W. G., Reidy J., Holt P. R., Krajewski S., Reed J. C., Blaser M. J., Moss S. F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997 Oct 20;239(2):626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehsel K., Kröncke K. D., Kolb-Bachofen V. Assay for detection of nitric oxide-induced apoptosis. Methods Enzymol. 1996;269:426–434. doi: 10.1016/s0076-6879(96)69043-0. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994 Feb;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fändriks L., von Bothmer C., Johansson B., Holm M., Bölin I., Pettersson A. Water extract of Helicobacter pylori inhibits duodenal mucosal alkaline secretion in anesthetized rats. Gastroenterology. 1997 Nov;113(5):1570–1575. doi: 10.1053/gast.1997.v113.pm9352859. [DOI] [PubMed] [Google Scholar]
- Genaro A. M., Hortelano S., Alvarez A., Martínez C., Boscá L. Splenic B lymphocyte programmed cell death is prevented by nitric oxide release through mechanisms involving sustained Bcl-2 levels. J Clin Invest. 1995 Apr;95(4):1884–1890. doi: 10.1172/JCI117869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Talanian R. V., Billiar T. R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997 Dec 5;272(49):31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., de Vera M. E., Watkins S. C., Billiar T. R. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997 Jan 10;272(2):1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- Kuo M. L., Chen C. W., Jee S. H., Chuang S. E., Cheng A. L. Transforming growth factor beta1 attenuates ceramide-induced CPP32/Yama activation and apoptosis in human leukaemic HL-60 cells. Biochem J. 1997 Nov 1;327(Pt 3):663–667. doi: 10.1042/bj3270663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque D., Kiss J., Tankovic J., Flejou J. F., Delchier J. C., Whittle B. J. Induction of nitric oxide synthase in vivo and cell injury in rat duodenal epithelium by a water soluble extract of Helicobacter pylori. Br J Pharmacol. 1998 Mar;123(6):1073–1078. doi: 10.1038/sj.bjp.0701706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Collazo E., Mateo J., Miras-Portugal M. T., Boscá L. Requirement of nitric oxide and calcium mobilization for the induction of apoptosis in adrenal vascular endothelial cells. FEBS Lett. 1997 Aug 11;413(1):124–128. doi: 10.1016/s0014-5793(97)00893-4. [DOI] [PubMed] [Google Scholar]
- Maeda H., Akaike T., Yoshida M., Suga M. Multiple functions of nitric oxide in pathophysiology and microbiology: analysis by a new nitric oxide scavenger. J Leukoc Biol. 1994 Nov;56(5):588–592. doi: 10.1002/jlb.56.5.588. [DOI] [PubMed] [Google Scholar]
- Mannick E. E., Bravo L. E., Zarama G., Realpe J. L., Zhang X. J., Ruiz B., Fontham E. T., Mera R., Miller M. J., Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996 Jul 15;56(14):3238–3243. [PubMed] [Google Scholar]
- McKnight G. M., Smith L. M., Drummond R. S., Duncan C. W., Golden M., Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997 Feb;40(2):211–214. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer U. K., Reimer D. M., Brüne B. Protease activation during nitric oxide-induced apoptosis: comparison between poly(ADP-ribose) polymerase and U1-70kDa cleavage. Eur J Pharmacol. 1998 May 22;349(2-3):333–343. doi: 10.1016/s0014-2999(98)00189-7. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Calam J., Agarwal B., Wang S., Holt P. R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996 Apr;38(4):498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. J., Hanson P. J., Whittle B. J. Localization of constitutive isoforms of nitric oxide synthase in the gastric glandular mucosa of the rat. Cell Tissue Res. 1996 Jul;285(1):157–163. doi: 10.1007/s004410050631. [DOI] [PubMed] [Google Scholar]
- Price K., Hanson P. Constitutive nitric oxide synthases in rat gastric mucosa: subcellular distribution, relative activity and different carboxyl-terminal antigenicity of the neuronal form compared with cerebellum. Digestion. 1998 Jul-Aug;59(4):308–313. doi: 10.1159/000007507. [DOI] [PubMed] [Google Scholar]
- Sandau K., Pfeilschifter J., Brüne B. The balance between nitric oxide and superoxide determines apoptotic and necrotic death of rat mesangial cells. J Immunol. 1997 May 15;158(10):4938–4946. [PubMed] [Google Scholar]
- Shen Y. H., Wang X. L., Wilcken D. E. Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett. 1998 Aug 14;433(1-2):125–131. doi: 10.1016/s0014-5793(98)00844-8. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Abrahamson T. D., Soper B. D. The role of cyclic guanylate monophosphate in nitric oxide-induced injury to rat small intestinal epithelial cells. J Pharmacol Exp Ther. 1998 Mar;284(3):929–933. [PubMed] [Google Scholar]
- Teshima S., Rokutan K., Nikawa T., Kishi K. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology. 1998 Nov;115(5):1186–1196. doi: 10.1016/s0016-5085(98)70090-3. [DOI] [PubMed] [Google Scholar]
- Tripp M. A., Tepperman B. L. Role of calcium in nitric oxide-mediated injury to rat gastric mucosal cells. Gastroenterology. 1996 Jul;111(1):65–72. doi: 10.1053/gast.1996.v111.pm8698226. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Reuter B., Cicala C., McKnight W., Grisham M. B., Cirino G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994 Jul;107(1):173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Wallach D. Cell death induction by TNF: a matter of self control. Trends Biochem Sci. 1997 Apr;22(4):107–109. doi: 10.1016/s0968-0004(97)01015-3. [DOI] [PubMed] [Google Scholar]
- Wilson K. T., Ramanujam K. S., Mobley H. L., Musselman R. F., James S. P., Meltzer S. J. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996 Dec;111(6):1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- Yanaka A., Muto H., Fukutomi H., Ito S., Silen W. Role of nitric oxide in restitution of injured guinea pig gastric mucosa in vitro. Am J Physiol. 1995 Jun;268(6 Pt 1):G933–G942. doi: 10.1152/ajpgi.1995.268.6.G933. [DOI] [PubMed] [Google Scholar]