Abstract
BACKGROUND—The development of colitis in interleukin 10 (IL-10) deficient mice, together with the known anti-inflammatory and immunomodulatory properties of this cytokine have prompted consideration of IL-10 as a treatment for inflammatory bowel disease (IBD). However, studies using hrIL-10 in IBD models have yielded inconsistent results. AIMS—To examine the therapeutic potential of overexpressing the IL-10 gene before and after the induction of experimental colitis in rats. METHODS—Gene transfer was achieved by intraperitoneal injection of non-replicating human type 5 adenovirus bearing the IL-10 gene, either 24 hours before or one hour after intrarectal administration of dinitrobenzene sulphonic acid in rats. Colonic damage and inflammation was assessed macroscopically and by measuring myeloperoxidase activity and leukotriene B4 concentrations. RESULTS—Gene transfer increased IL-10 protein in serum for up to six days. IL-10 gene transfer prior to colitis improved colitis macroscopically and histologically, and significantly reduced colonic myeloperoxidase activity and leukotriene B4 concentrations. In contrast, IL-10 gene transfer after the onset of colitis had no beneficial effect. CONCLUSIONS—Gene therapy using an adenovirus-IL-10 construct was successful in preventing but not in reversing experimental colitis in the rat. Keywords: gene therapy; colitis; interleukin 10; adenovirus vector; leukotrienes; inflammatory bowel disease; maintenance therapy
Full Text
The Full Text of this article is available as a PDF (136.1 KB).
Figure 1 .
Luciferase activity following administration of Ad5Luc3 or Ad5IL-10. Results represent mean (SEM) from each group (n=8 rats for each group).
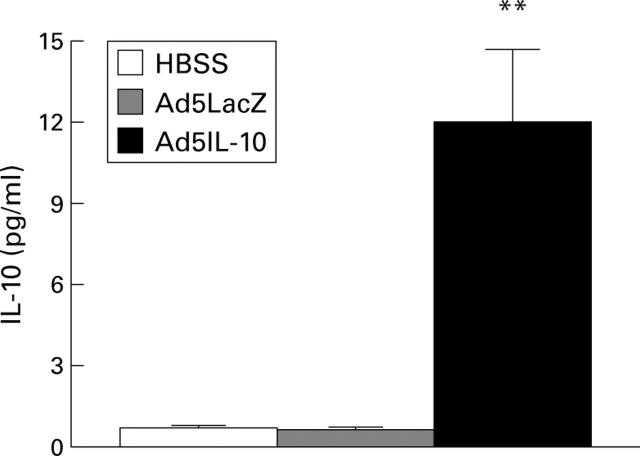
Figure 2 .
Serum IL-10 protein concentrations after administration of HBSS, Ad5LacZ, or Ad5IL-10. Results represent mean (SEM) from each group (HBSS, n=4; Ad5LacZ, n=4; Ad5IL-10, n=8). **Significantly different from controls (p<0.01).
Figure 3 .
Macroscopic damage scores six days after dinitrobenzene sulphonic acid (DNB) induced colitis. Results represent mean (SEM) from each group (ethanol group, n=6; HBSS, Ad5LacZ, and Ad5IL-10, n=8). *Significantly different from Ad5LacZ (p<0.05).
Figure 4 .
Histological damage scores six days after dinitrobenzene sulphonic acid (DNB) induced colitis. Results represent mean (SEM) from each group (ethanol group, n=6; HBSS, Ad5LacZ, and Ad5IL-10, n=8). *Significantly different from Ad5LacZ (p<0.05).
Figure 5 .
Myeloperoxidase activity six days after dinitrobenzene sulphonic acid (DNB) induced colitis. Results represent mean (SEM) from each group (ethanol group, n=6; HBSS, Ad5LacZ, and Ad5IL-10, n=8). *Significantly different from Ad5LacZ (p<0.05).
Figure 6 .
Effect of pretreatment of rats with Ad5IL-10 gene transfer on leukotriene (LT) B4 concentrations in the distal colon of rats six days after dinitrobenzene sulphonic acid (DNB) induced colitis. Results represent mean (SEM) from each group (ethanol group, n=6; HBSS, Ad5LacZ, and Ad5IL-10, n=8). *Significantly different from Ad5LacZ (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. J., Davidson N., Kühn R., Müller W., Menon S., Holland G., Thompson-Snipes L., Leach M. W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996 Aug 15;98(4):1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Wallace J. L., Whittle B. J. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988 Aug;25(1-2):115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- Brown G. R., Thiele D. L., Silva M., Beutler B. Adenoviral vectors given intravenously to immunocompromised mice yield stable transduction of the colonic epithelium. Gastroenterology. 1997 May;112(5):1586–1594. doi: 10.1016/s0016-5085(97)70040-4. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993 Dec 1;178(6):2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff A. E., Granowitz E. V., Shapiro L., Vannier E., Lonnemann G., Angel J. B., Kennedy J. S., Rabson A. R., Wolff S. M., Dinarello C. A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995 May 15;154(10):5492–5499. [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Duchini A., Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992 Jul;103(1):65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- Drazan K. E., Wu L., Bullington D., Shaked A. Viral IL-10 gene therapy inhibits TNF-alpha and IL-1 beta, not IL-6, in the newborn endotoxemic mouse. J Pediatr Surg. 1996 Mar;31(3):411–414. doi: 10.1016/s0022-3468(96)90749-6. [DOI] [PubMed] [Google Scholar]
- During M. J., Xu R., Young D., Kaplitt M. G., Sherwin R. S., Leone P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat Med. 1998 Oct;4(10):1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- Hamilton T. E., McClane S. J., Baldwin S., Burke C., Patel H., Rombeau J. L., Raper S. E. Efficient adenoviral-mediated murine neonatal small intestinal gene transfer is dependent on alpha(v) integrin expression. J Pediatr Surg. 1997 Dec;32(12):1695–1703. doi: 10.1016/s0022-3468(97)90508-x. [DOI] [PubMed] [Google Scholar]
- Herfarth H. H., Mohanty S. P., Rath H. C., Tonkonogy S., Sartor R. B. Interleukin 10 suppresses experimental chronic, granulomatous inflammation induced by bacterial cell wall polymers. Gut. 1996 Dec;39(6):836–845. doi: 10.1136/gut.39.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam C. M., Vallance B. A., Kumar A., Addison C. L., Graham F. L., Gauldie J., Collins S. M. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J Clin Invest. 1997 Dec 1;100(11):2766–2776. doi: 10.1172/JCI119823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iademarco M. F., Barks J. L., Dean D. C. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995 Jan;95(1):264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasama T., Strieter R. M., Lukacs N. W., Burdick M. D., Kunkel S. L. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994 Apr 1;152(7):3559–3569. [PubMed] [Google Scholar]
- Keel M., Ungethüm U., Steckholzer U., Niederer E., Hartung T., Trentz O., Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997 Nov 1;90(9):3356–3363. [PubMed] [Google Scholar]
- Krakauer T. IL-10 inhibits the adhesion of leukocytic cells to IL-1-activated human endothelial cells. Immunol Lett. 1995 Feb;45(1-2):61–65. doi: 10.1016/0165-2478(94)00226-h. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Macdonald T. T. Viral vectors expressing immunoregulatory cytokines to treat inflammatory bowel disease. Gut. 1998 Apr;42(4):460–461. doi: 10.1136/gut.42.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Leal-Berumen I., Nielsen L., Glibetic M., Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996 Feb 15;97(4):1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Piedimonte G., Pickles R. J., Lehmann J. R., McCarty D., Costa D. L., Boucher R. C. Replication-deficient adenoviral vector for gene transfer potentiates airway neurogenic inflammation. Am J Respir Cell Mol Biol. 1997 Mar;16(3):250–258. doi: 10.1165/ajrcmb.16.3.9070609. [DOI] [PubMed] [Google Scholar]
- Ribbons K. A., Thompson J. H., Liu X., Pennline K., Clark D. A., Miller M. J. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur J Pharmacol. 1997 Apr 4;323(2-3):245–254. doi: 10.1016/s0014-2999(97)00017-4. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Sferra T. J., McNeely D., Johnson P. R. Gene transfer to the intestinal tract: a new approach using selective injection of the superior mesenteric artery. Hum Gene Ther. 1997 Apr 10;8(6):681–687. doi: 10.1089/hum.1997.8.6-681. [DOI] [PubMed] [Google Scholar]
- Sironi M., Muñoz C., Pollicino T., Siboni A., Sciacca F. L., Bernasconi S., Vecchi A., Colotta F., Mantovani A. Divergent effects of interleukin-10 on cytokine production by mononuclear phagocytes and endothelial cells. Eur J Immunol. 1993 Oct;23(10):2692–2695. doi: 10.1002/eji.1830231046. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990 Apr;258(4 Pt 1):G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Le T., Carter L., Appleyard C. B., Beck P. L. Hapten-induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods. 1995 Aug;33(4):237–239. doi: 10.1016/1056-8719(95)00001-x. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., MacNaughton W. K., Morris G. P., Beck P. L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., McKnight W., Asfaha S., Liu D. Y. Reduction of acute and reactivated colitis in rats by an inhibitor of neutrophil activation. Am J Physiol. 1998 May;274(5 Pt 1):G802–G808. doi: 10.1152/ajpgi.1998.274.5.G802. [DOI] [PubMed] [Google Scholar]
- Xing Z., Ohkawara Y., Jordana M., Graham F. L., Gauldie J. Adenoviral vector-mediated interleukin-10 expression in vivo: intramuscular gene transfer inhibits cytokine responses in endotoxemia. Gene Ther. 1997 Feb;4(2):140–149. doi: 10.1038/sj.gt.3300371. [DOI] [PubMed] [Google Scholar]
- de Vries J. E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995 Oct;27(5):537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer S. J., Elson C. O., Fedorak R. N. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997 Aug;113(2):383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]