Abstract
BACKGROUND/AIMS—Biosynthesis of carbohydrate structures is tissue specific and developmentally regulated by glycosyltransferases such as fucosyltransferases, sialyltransferases, and N-acetylgluco- saminyltransferases. During carcinogenesis, aberrant glycosylation leads to the development of tumour subpopulations with different adhesion properties. Therefore alterations in glycosyltransferase mRNA expression in colorectal carcinomas were examined by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR). METHODS—Colorectal carcinoma specimens were classified and characterised according to the WHO/UICC system. Expression of fucosyltransferases FT-I, FT-III, FT-IV, FT-V, FT-VI, and FT-VII, sialyltransferases ST3Gal-I, ST3Gal-III, ST3Gal-IV, and ST6Gal-I, β1,4-galacto- syltransferase, and β1,6-Nacetylgluco- saminyltransferase V (GNT-V) was screened simultaneously in extracts of 22 homogenised tumour specimens by RT-PCR and compared with corresponding mucosa from each patient. Also 12 adenomas and 17 liver metastases of colorectal carcinomas were examined. RESULTS—GNT-V expression was enhanced in colorectal adenomas (p = 0.039), carcinomas (p<0.001), and liver metastases of colorectal carcinomas (p<0.001). Also, expression of fucosyltransferase FT-IV was increased in colorectal adenomas (p = 0.039) and carcinomas (p<0.001). In addition, fucosyltransferase FT-I (p<0.001) and sialyltransferases ST6Gal-I (p = 0.004) and ST3Gal-III (p = 0.001) showed increased expression in carcinoma specimens. On the other hand, fucosyltransferase FT-III was less abundantly expressed in carcinomas exhibiting distant metastases (p = 0.046) and in highly invasive tumours (p = 0.041). CONCLUSIONS—Glycosyltransferase mRNA expression is significantly altered in colorectal adenomas and carcinomas isolated from surgical specimens. RT-PCR determination of specific glycosyltransferases may be helpful for earlier detection of carcinomas and for tumour prognosis. Keywords: colorectal carcinoma; adenomas; liver metastasis; glycosyltransferases; tumour prognosis; mRNA expression
Full Text
The Full Text of this article is available as a PDF (195.7 KB).
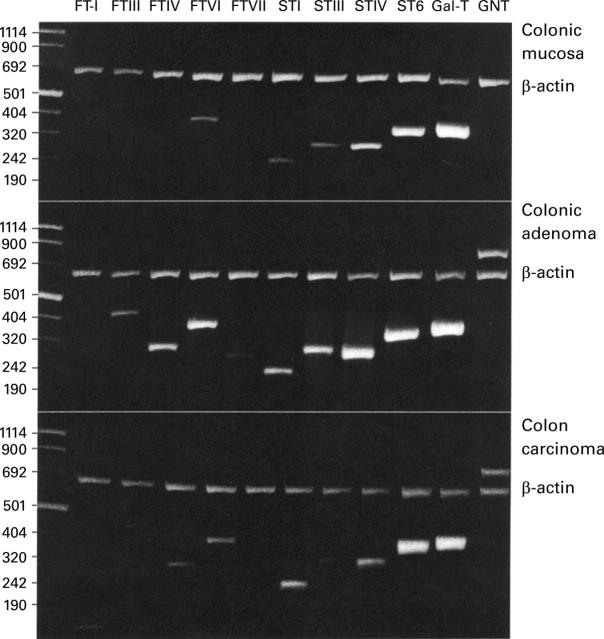
Figure 1 .
Glycosyltransferase expression in human colorectal tissue from case 3. Fluorescence of electrophoretically resolved, ethidium bromide stained polymerase chain reaction (PCR) products of β-actin and glycosyltransferase target sequences from colon mucosa, colon adenoma tissue, and colon carcinoma tissue is shown. Glycosyltransferases were amplified as described in Materials and methods. The upper band shows the reaction product of β-actin amplification. All amplicons were sequenced and compared with published sequences. First lane, molecular mass markers (bp). FT-I, H blood group α1,2-fucosyltransferase I; FTIII-VII, fucosyltransferases III-VII; STI-IV, sialyltransferases ST3Gal-I-IV; ST6, sialyltransferase ST6Gal-I; Gal-T, β1,4-galactosyltransferase; GNT, β1,6-N-acetylglucosaminyltransferase V.
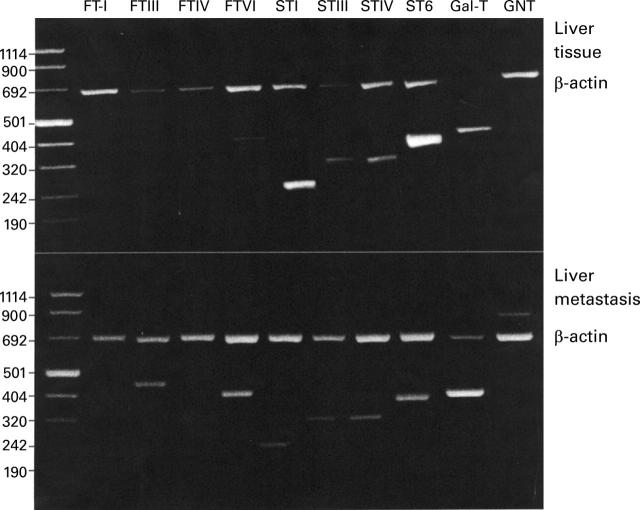
Figure 2 .
Glycosyltransferase expression in liver and liver metastases of a human colorectal carcinoma from case 35. Fluorescence of electrophoretically resolved, ethidium bromide stained polymerase chain reaction (PCR) products of β-actin and glycosyltransferase target sequences from normal liver tissue and liver metastases is shown. Glycosyltransferases were amplified as described in Materials and methods. The upper band shows the reaction product of β-actin amplification. All amplicons were sequenced and compared with published sequences. First lane, molecular mass markers (bp). FT-I, H blood group α1,2-fucosyltransferase I; FTIII-VII, fucosyltransferases III-VII; STI-IV, sialyltransferases ST3Gal-I-IV; ST6, sialyltransferase ST6Gal-I; Gal-T, β1,4-galactosyltransferase; GNT, β1,6-N-acetylglucosaminyltransferase V.
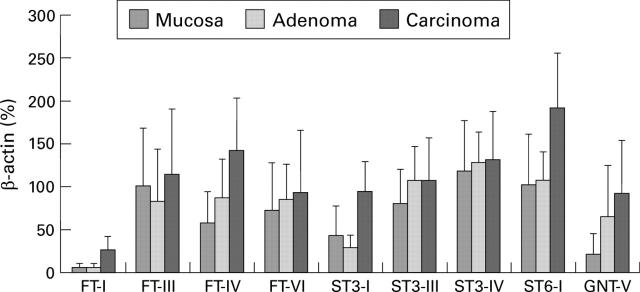
Figure 3 .
Glycosyltransferase expression in human colorectal tissue. For semiquantitative analysis of the reverse transcription-polymerase chain reaction data, fluorescence of each sample was compared with that of β-actin co-amplified in the same tube. Standard deviations describe tumour heterogeneity not the accuracy of the assay system. FT-I, H blood group α1,2-fucosyltransferase I; FTIII-VI, fucosyltransferases III-VI; STI-IV, sialyltransferases ST3Gal-I-IV; ST6-I, sialyltransferase ST6Gal-I; GNT-V, β1,6-N-acetylglucosaminyltransferase V.
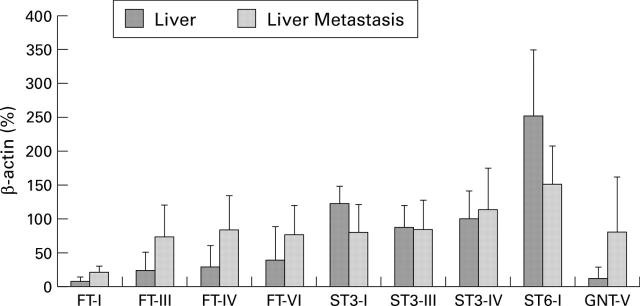
Figure 4 .
Glycosyltransferase expression in liver metastases of human colorectal carcinomas. For semiquantitative analysis of the reverse transcription-polymerase chain reaction data, fluorescence of each sample was compared with that of β-actin co-amplified within the same tube. Standard deviations describe tumour heterogeneity not the accuracy of the assay system. FT-I, H blood group α1,2-fucosyltransferase I; FTIII-VI, fucosyltransferases III-VI; STI-IV, sialyltransferases ST3Gal-I-IV; ST6-I, sialyltransferase ST6Gal-I; GNT-V, β1,6-N-acetylglucosaminyltransferase V.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch J., Brossmer R., Kemmner W., Schlag P. Preparation and characterization of differently aggregated colorectal carcinoma cell subpopulations from surgical specimens. Cancer Detect Prev. 1998;22(4):319–329. doi: 10.1046/j.1525-1500.1998.cdoa42.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F., Malagolini N., Serafini-Cessi F. Enhanced CMP-NeuAc:Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity of human colon cancer xenografts in athymic nude mice and of xenograft-derived cell lines. Int J Cancer. 1992 Jan 21;50(2):325–330. doi: 10.1002/ijc.2910500227. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F., Malagolini N., di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Increased CMP-NeuAc:Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity in human colorectal cancer tissues. Int J Cancer. 1989 Sep 15;44(3):434–439. doi: 10.1002/ijc.2910440309. [DOI] [PubMed] [Google Scholar]
- Demetriou M., Nabi I. R., Coppolino M., Dedhar S., Dennis J. W. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J Cell Biol. 1995 Jul;130(2):383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. N-linked oligosaccharide processing and tumor cell biology. Semin Cancer Biol. 1991 Dec;2(6):411–420. [PubMed] [Google Scholar]
- Fernandes B., Sagman U., Auger M., Demetrio M., Dennis J. W. Beta 1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res. 1991 Jan 15;51(2):718–723. [PubMed] [Google Scholar]
- Gessner P., Riedl S., Quentmaier A., Kemmner W. Enhanced activity of CMP-neuAc:Gal beta 1-4GlcNAc:alpha 2,6-sialyltransferase in metastasizing human colorectal tumor tissue and serum of tumor patients. Cancer Lett. 1993 Dec 20;75(3):143–149. doi: 10.1016/0304-3835(93)90056-f. [DOI] [PubMed] [Google Scholar]
- Goupille C., Hallouin F., Meflah K., Le Pendu J. Increase of rat colon carcinoma cells tumorigenicity by alpha(1-2) fucosyltransferase gene transfection. Glycobiology. 1997 Mar;7(2):221–229. doi: 10.1093/glycob/7.2.221. [DOI] [PubMed] [Google Scholar]
- Grundmann U., Nerlich C., Rein T., Zettlmeissl G. Complete cDNA sequence encoding human beta-galactoside alpha-2,6-sialyltransferase. Nucleic Acids Res. 1990 Feb 11;18(3):667–667. doi: 10.1093/nar/18.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hanski C., Klussmann E., Wang J., Böhm C., Ogorek D., Hanski M. L., Krüger-Krasagakes S., Eberle J., Schmitt-Gräff A., Riecken E. O. Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue. Glycoconj J. 1996 Oct;13(5):727–733. doi: 10.1007/BF00702336. [DOI] [PubMed] [Google Scholar]
- Hiraiwa N., Dohi T., Kawakami-Kimura N., Yumen M., Ohmori K., Maeda M., Kannagi R. Suppression of sialyl Lewis X expression and E-selectin-mediated cell adhesion in cultured human lymphoid cells by transfection of antisense cDNA of an alpha1-->3 fucosyltransferase (Fuc-T VII). J Biol Chem. 1996 Dec 6;271(49):31556–31561. doi: 10.1074/jbc.271.49.31556. [DOI] [PubMed] [Google Scholar]
- Ito H., Hiraiwa N., Sawada-Kasugai M., Akamatsu S., Tachikawa T., Kasai Y., Akiyama S., Ito K., Takagi H., Kannagi R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int J Cancer. 1997 May 16;71(4):556–564. doi: 10.1002/(sici)1097-0215(19970516)71:4<556::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997 Aug;14(5):577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- Kelly R. J., Rouquier S., Giorgi D., Lennon G. G., Lowe J. B. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995 Mar 3;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- Kemmner W., Hohaus K., Schlag P. M. Inhibition of Gal beta1, 4GlcNAc alpha2,6 sialyltransferase expression by antisense-oligodeoxynucleotides. FEBS Lett. 1997 Jun 16;409(3):347–350. doi: 10.1016/s0014-5793(97)00543-7. [DOI] [PubMed] [Google Scholar]
- Kemmner W., Krück D., Schlag P. Different sialyltransferase activities in human colorectal carcinoma cells from surgical specimens detected by specific glycoprotein and glycolipid acceptors. Clin Exp Metastasis. 1994 May;12(3):245–254. doi: 10.1007/BF01753893. [DOI] [PubMed] [Google Scholar]
- Kitagawa H., Paulson J. C. Cloning and expression of human Gal beta 1,3(4)GlcNAc alpha 2,3-sialyltransferase. Biochem Biophys Res Commun. 1993 Jul 15;194(1):375–382. doi: 10.1006/bbrc.1993.1830. [DOI] [PubMed] [Google Scholar]
- Kitagawa H., Paulson J. C. Cloning of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 1994 Jan 14;269(2):1394–1401. [PubMed] [Google Scholar]
- Korczak B., Goss P., Fernandez B., Baker M., Dennis J. W. Branching N-linked oligosaccharides in breast cancer. Adv Exp Med Biol. 1994;353:95–104. doi: 10.1007/978-1-4615-2443-4_10. [DOI] [PubMed] [Google Scholar]
- Koszdin K. L., Bowen B. R. The cloning and expression of a human alpha-1,3 fucosyltransferase capable of forming the E-selectin ligand. Biochem Biophys Res Commun. 1992 Aug 31;187(1):152–157. doi: 10.1016/s0006-291x(05)81472-x. [DOI] [PubMed] [Google Scholar]
- Kudo T., Ikehara Y., Togayachi A., Morozumi K., Watanabe M., Nakamura M., Nishihara S., Narimatsu H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Invest. 1998 Jul;78(7):797–811. [PubMed] [Google Scholar]
- Kukowska-Latallo J. F., Larsen R. D., Nair R. P., Lowe J. B. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. 1990 Aug;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Kumar R., Potvin B., Muller W. A., Stanley P. Cloning of a human alpha(1,3)-fucosyltransferase gene that encodes ELFT but does not confer ELAM-1 recognition on Chinese hamster ovary cell transfectants. J Biol Chem. 1991 Nov 15;266(32):21777–21783. [PubMed] [Google Scholar]
- Larsen R. D., Ernst L. K., Nair R. P., Lowe J. B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6674–6678. doi: 10.1073/pnas.87.17.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. P., Zuber C., Heitz P. U., Roth J. Cytochemical staining for beta 1,6 branching of asparagine-linked oligosaccharides in variants of metastatic human colon carcinoma cells. Am J Pathol. 1994 Aug;145(2):470–480. [PMC free article] [PubMed] [Google Scholar]
- Liepkalns V. A., Eboué D., Beringer T., Sabri A., Icard-Liepkalns C. Repression of the Lewis fucosyl transferase by retinoic acid increases apical sialosyl Lewis(a) secretion in colorectal carcinoma cultures. J Cell Biochem. 1995 Jul;58(3):292–304. doi: 10.1002/jcb.240580304. [DOI] [PubMed] [Google Scholar]
- Lowe J. B., Kukowska-Latallo J. F., Nair R. P., Larsen R. D., Marks R. M., Macher B. A., Kelly R. J., Ernst L. K. Molecular cloning of a human fucosyltransferase gene that determines expression of the Lewis x and VIM-2 epitopes but not ELAM-1-dependent cell adhesion. J Biol Chem. 1991 Sep 15;266(26):17467–17477. [PubMed] [Google Scholar]
- Masri K. A., Appert H. E., Fukuda M. N. Identification of the full-length coding sequence for human galactosyltransferase (beta-N-acetylglucosaminide: beta 1,4-galactosyltransferase). Biochem Biophys Res Commun. 1988 Dec 15;157(2):657–663. doi: 10.1016/s0006-291x(88)80300-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Muramatsu H., Shimotakahara T., Yanagi M., Nishijima H., Mitani N., Baba K., Muramatsu T., Shimazu H. Correlation of expression of ABH blood group carbohydrate antigens with metastatic potential in human lung carcinomas. Cancer. 1993 Jul 1;72(1):75–81. doi: 10.1002/1097-0142(19930701)72:1<75::aid-cncr2820720116>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J., Kemmner W., Brossmer R. Sialic acid dependent cell adhesion to collagen IV correlates with in vivo tumorigenicity of the human colon carcinoma sublines HCT116, HCT116a and HCT116b. Biochem Biophys Res Commun. 1990 Sep 14;171(2):860–866. doi: 10.1016/0006-291x(90)91225-h. [DOI] [PubMed] [Google Scholar]
- Natsuka S., Gersten K. M., Zenita K., Kannagi R., Lowe J. B. Molecular cloning of a cDNA encoding a novel human leukocyte alpha-1,3-fucosyltransferase capable of synthesizing the sialyl Lewis x determinant. J Biol Chem. 1994 Jun 17;269(24):16789–16794. [PubMed] [Google Scholar]
- O'Hanlon T. P., Lau K. M., Wang X. C., Lau J. T. Tissue-specific expression of beta-galactoside alpha-2,6-sialyltransferase. Transcript heterogeneity predicts a divergent polypeptide. J Biol Chem. 1989 Oct 15;264(29):17389–17394. [PubMed] [Google Scholar]
- Oriol R., Mollicone R., Coullin P., Dalix A. M., Candelier J. J. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl. 1992;27:28–38. [PubMed] [Google Scholar]
- Orntoft T. F., Greenwell P., Clausen H., Watkins W. M. Regulation of the oncodevelopmental expression of type 1 chain ABH and Lewis(b) blood group antigens in human colon by alpha-2-L-fucosylation. Gut. 1991 Mar;32(3):287–293. doi: 10.1136/gut.32.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orntoft T. F., Meldgaard P., Pedersen B., Wolf H. The blood group ABO gene transcript is down-regulated in human bladder tumors and growth-stimulated urothelial cell lines. Cancer Res. 1996 Mar 1;56(5):1031–1036. [PubMed] [Google Scholar]
- Orntoft T. F., Vestergaard E. M. Clinical aspects of altered glycosylation of glycoproteins in cancer. Electrophoresis. 1999 Feb;20(2):362–371. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Weinstein J., Schauer A. Tissue-specific expression of sialyltransferases. J Biol Chem. 1989 Jul 5;264(19):10931–10934. [PubMed] [Google Scholar]
- Pilatte Y., Bignon J., Lambré C. R. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993 Jun;3(3):201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchi M. A., Hebbar M., Hornez L., Harduin-Lepers A., Peyrat J. P., Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998 Sep 15;58(18):4066–4070. [PubMed] [Google Scholar]
- Saito H., Nishikawa A., Gu J., Ihara Y., Soejima H., Wada Y., Sekiya C., Niikawa N., Taniguchi N. cDNA cloning and chromosomal mapping of human N-acetylglucosaminyltransferase V+. Biochem Biophys Res Commun. 1994 Jan 14;198(1):318–327. doi: 10.1006/bbrc.1994.1045. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Kurata K., Funayama K., Nagata M., Watanabe E., Ohta S., Hanai N., Nishi T. Expression cloning of a novel alpha 1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis x carbohydrate determinants in leukocytes. J Biol Chem. 1994 May 20;269(20):14730–14737. [PubMed] [Google Scholar]
- Sasaki K., Watanabe E., Kawashima K., Sekine S., Dohi T., Oshima M., Hanai N., Nishi T., Hasegawa M. Expression cloning of a novel Gal beta (1-3/1-4) GlcNAc alpha 2,3-sialyltransferase using lectin resistance selection. J Biol Chem. 1993 Oct 25;268(30):22782–22787. [PubMed] [Google Scholar]
- Schauer R. Biosynthesis and function of N- and O-substituted sialic acids. Glycobiology. 1991 Nov;1(5):449–452. doi: 10.1093/glycob/1.5.449. [DOI] [PubMed] [Google Scholar]
- Seelentag W. K., Li W. P., Schmitz S. F., Metzger U., Aeberhard P., Heitz P. U., Roth J. Prognostic value of beta1,6-branched oligosaccharides in human colorectal carcinoma. Cancer Res. 1998 Dec 1;58(23):5559–5564. [PubMed] [Google Scholar]
- Sun J., Thurin J., Cooper H. S., Wang P., Mackiewicz M., Steplewski Z., Blaszczyk-Thurin M. Elevated expression of H type GDP-L-fucose:beta-D-galactoside alpha-2-L-fucosyltransferase is associated with human colon adenocarcinoma progression. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5724–5728. doi: 10.1073/pnas.92.12.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Yoshimura M., Miyoshi E., Ihara Y., Nishikawa A., Fujii S. Remodeling of cell surface glycoproteins by N-acetylglucosaminyltransferase III gene transfection: modulation of metastatic potentials and down regulation of hepatitis B virus replication. Glycobiology. 1996 Oct;6(7):691–694. doi: 10.1093/glycob/6.7.691. [DOI] [PubMed] [Google Scholar]
- Weston B. W., Nair R. P., Larsen R. D., Lowe J. B. Isolation of a novel human alpha (1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group alpha (1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J Biol Chem. 1992 Feb 25;267(6):4152–4160. [PubMed] [Google Scholar]
- Whitehouse C., Burchell J., Gschmeissner S., Brockhausen I., Lloyd K. O., Taylor-Papadimitriou J. A transfected sialyltransferase that is elevated in breast cancer and localizes to the medial/trans-Golgi apparatus inhibits the development of core-2-based O-glycans. J Cell Biol. 1997 Jun 16;137(6):1229–1241. doi: 10.1083/jcb.137.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago K., Zenita K., Ginya H., Sawada M., Ohmori K., Okuma M., Kannagi R., Lowe J. B. Expression of alpha-(1,3)-fucosyltransferases which synthesize sialyl Le(x) and sialyl Le(a), the carbohydrate ligands for E- and P-selectins,in human malignant cell lines. Cancer Res. 1993 Nov 15;53(22):5559–5565. [PubMed] [Google Scholar]
- Zhang L., Zhou W., Velculescu V. E., Kern S. E., Hruban R. H., Hamilton S. R., Vogelstein B., Kinzler K. W. Gene expression profiles in normal and cancer cells. Science. 1997 May 23;276(5316):1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]