Abstract
BACKGROUND—TFF1 is a 6.5 kDa secreted protein that is expressed predominantly in normal gastric mucosa. It is coexpressed with mucins and it can form dimers via a free carboxy terminal cysteine residue. AIMS—To investigate the molecular forms of TFF1 that are present in normal human stomach and the association of the different molecular forms with mucus. SUBJECTS—All subjects had macroscopically normal stomachs at gastroscopy. None had a significant past medical history. METHODS—TFF1 was detected in normal gastric mucosa and adherent mucus by western transfer analysis after electrophoresis on reducing and non-reducing polyacrylamide gels. In some instances, proteins were fractionated by caesium chloride density gradient centrifugation prior to detection of TFF1. The location of TFF1 in gastric mucosa with an intact adherent mucus layer was assessed by immunohistochemistry. RESULTS—Three different molecular forms of TFF1 were detected: TFF1 monomer, TFF1 dimer, and a TFF1 complex with an apparent molecular mass of about 25 kDa. TFF1 was present at higher concentrations than realised previously. The TFF1 complex was present in the adherent mucus gel layer but while its interaction with mucin was destabilised by caesium chloride, the interaction between mucin and the TFF1 dimer was resistant to caesium chloride. CONCLUSIONS—Most of TFF1 in normal human gastric mucosa is present in a complex that is stabilised by a disulphide bond. TFF1 is intimately associated with mucus. The high concentration, colocalisation, and binding of TFF1 to gastric mucus strongly implicate TFF1 in gastric mucus function. Keywords: TFF peptide; pS2; gastric; mucin; disulphide bond; adherent mucus gel
Full Text
The Full Text of this article is available as a PDF (220.9 KB).
Figure 1 .
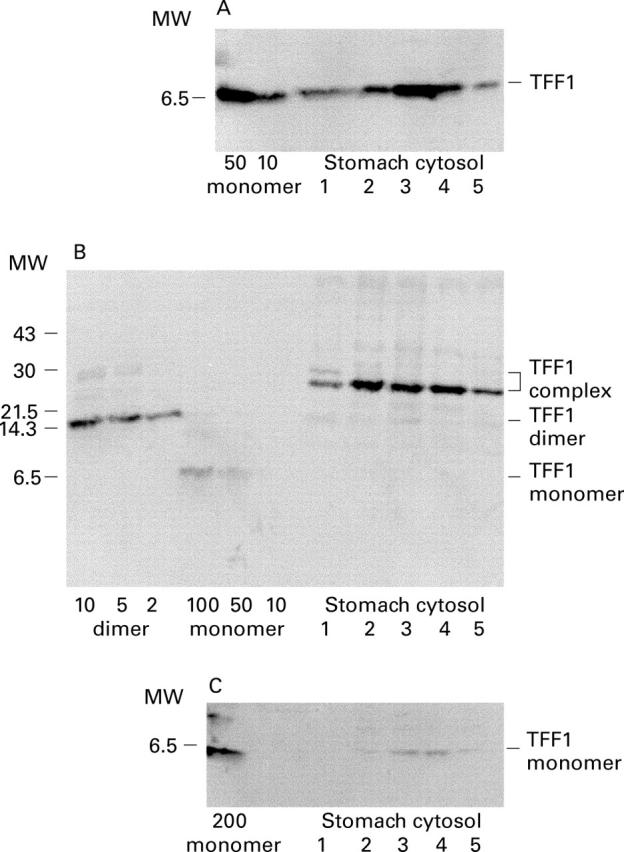
Different molecular forms of TFF1 present on normal human gastric mucosa. Cytosol was prepared from small biopsy specimens of gastric mucosa. Aliquots of 5 µl, or 10 µl for sample 1 in A and C, were electrophoresed on 10-35% (A and C) or 20% (B) polyacrylamide gels and the separated proteins transferred to PVDF membrane for 15 min (A and B) or 10 min (C) and then incubated with anti-TFF1 antisera. Prior to electrophoresis, the samples were boiled in sample buffer that did (A) or did not contain β-mercaptoethanol (B and C). Known amounts of recombinant TFF1 monomer or TFF1 dimer were included in the gels as indicated below in ng. The positions of molecular mass markers are shown on the left, and of mature TFF1 (A), TFF1 complex, TFF1 dimer, TFF1 monomer (B), and TFF1 monomer (C) on the right.
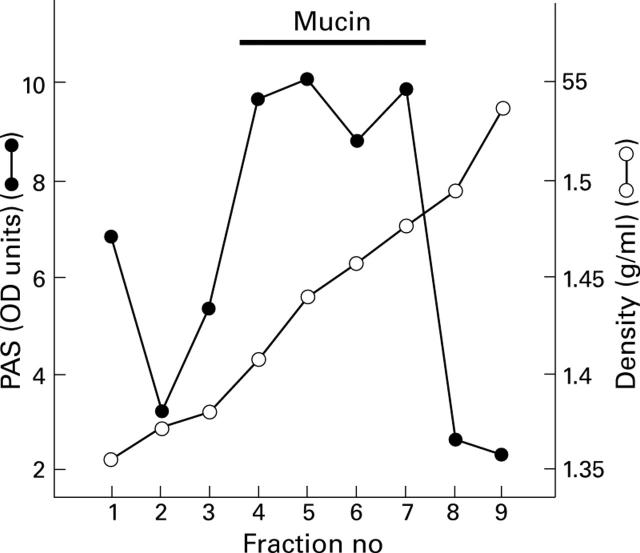
Figure 2 .
Fractionation of human gastric mucus by equilibrium density centrifugation in a caesium chloride gradient. Normal gastric mucus was prepared from 2 mm3 antral biopsy specimens, and CsCl was added to give a density of 1.42 g/ml. After centrifugation the gradient was divided into nine fractions of equal volume and the density of the fractions measured. The glycoprotein content of the fractions was measured as described previously.35
Figure 3 .
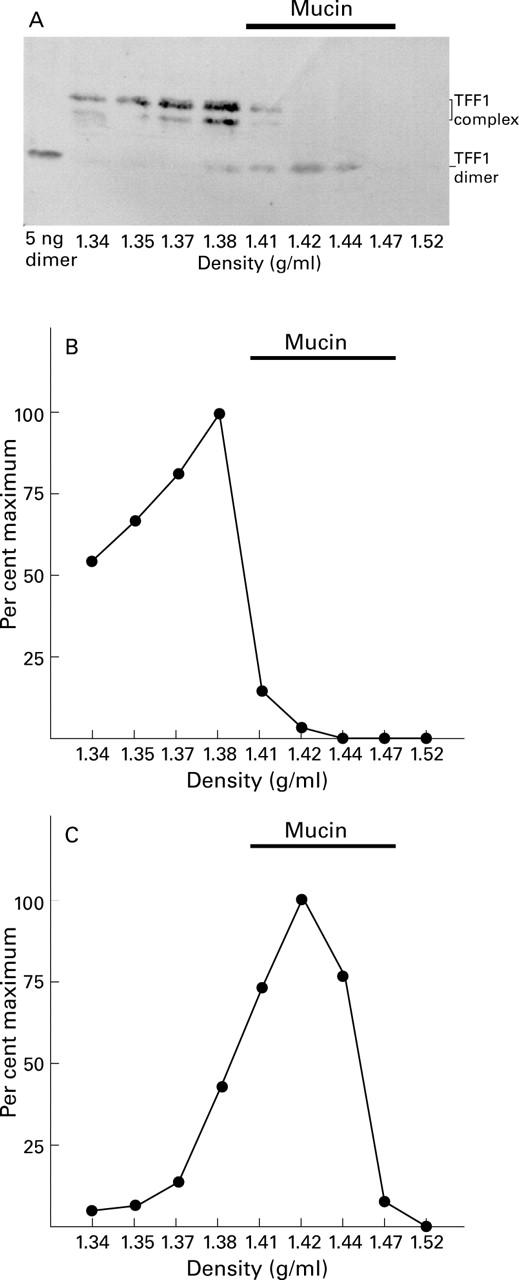
Association of different molecular forms of TFF1 with gastric mucin. Twelve 2 mm3 biopsy specimens of normal gastric mucosa were homogenised and centrifuged; caesium chloride was added to the supernatant to give a density of 1.42 g/ml. After centrifugation to equilibrium, the gradient was divided into nine equal fractions which were analysed for the presence of TFF1 under non-reducing conditions by western transfer. The resultant membrane is shown (A) with the positions of the TFF1 complex and TFF1 dimer indicated on the right hand side. Recombinant TFF1 dimer 5 ng was included in the gel. The intensity of the reaction with the different protein bands was quantified by image analysis and is expressed as a percentage of the maximum intensity for either the TFF1 complex (B) or the TFF1 dimer (C).
Figure 4 .
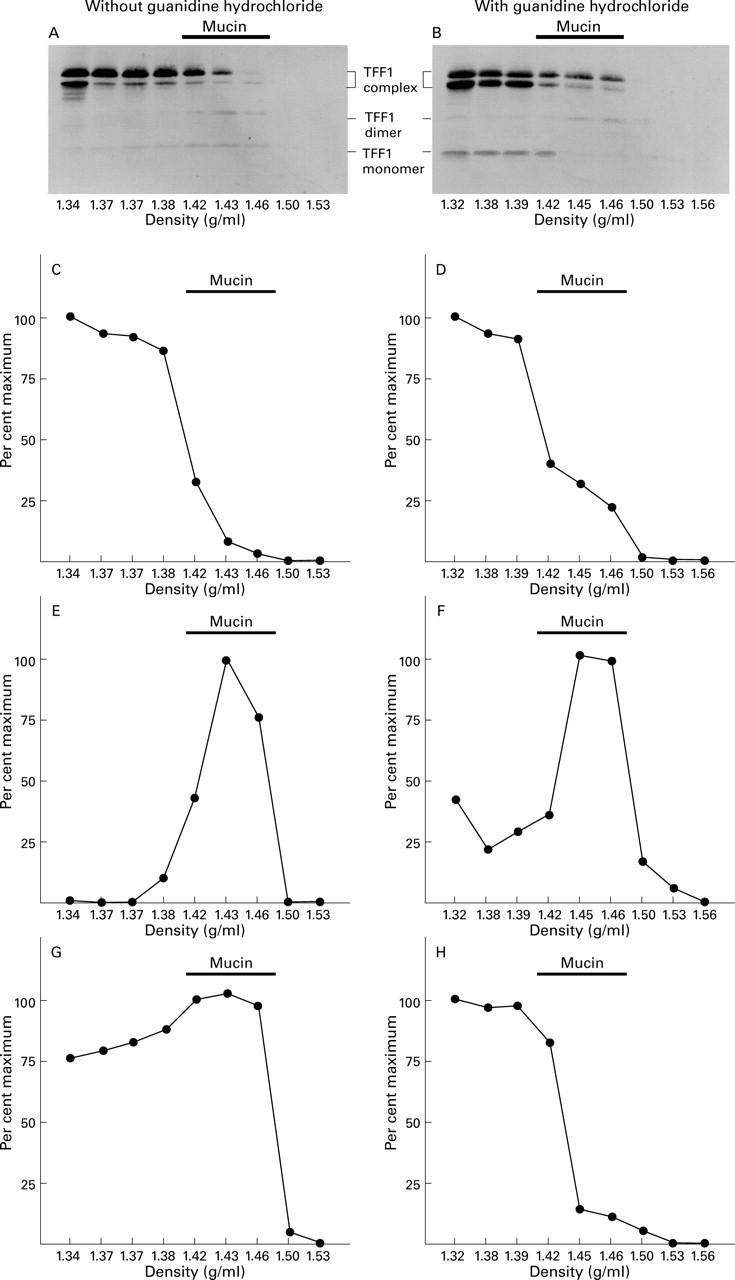
Stability of the interaction between TFF dimer and gastric mucin. Twenty four 2 mm3 biopsy specimens of normal antral mucosa were homogenised and centrifuged and the supernatant was divided into two halves. Caesium chloride was added to both to a final concentration of 1.42 g/ml, and guanidine hydrochloride to one half to a final concentration of 6 M. After centrifugation to equilibrium, the gradients were each divided into nine fractions of equal volume which were analysed for the presence of TFF1 by western transfer after electrophoresis under non-reducing conditions. The resultant membranes are shown (A and B) with the positions of the TFF1 complexes, TFF1 dimer, and TFF1 monomer indicated on the right. The intensity of the reaction with the TFF1 complex (C and D), TFF1 dimer (E and F), and TFF1 monomer (G and H) were quantified by image analysis and expressed as a percentage of the maximum intensity for each form.
Figure 5 .
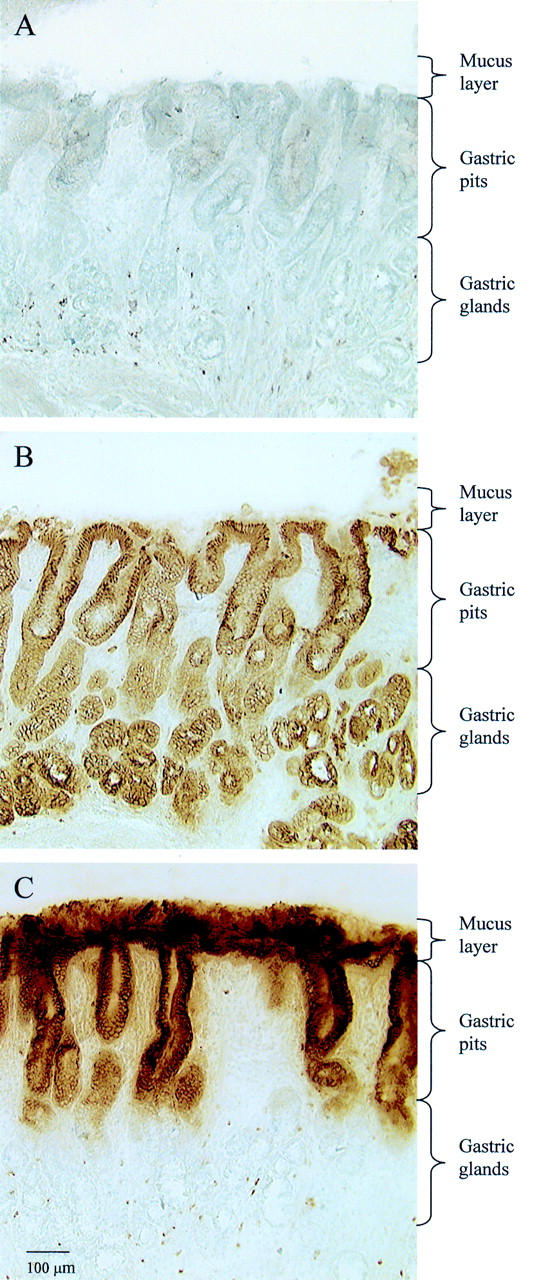
Distribution of TFF1 in situ in cryostat sections of normal human gastric antral mucosa. Sections were processed by immunohistochemistry to locate cytokeratins and TFF1. (A) No specific immunoreaction was obtained in the absence of primary antibodies. (B) There was an intense cytoplasmic immunoreaction of epithelial cells throughout the mucosa with antibodies to cytokeratins. (C) With anti-TFF1 antibodies, there was an intense immunoreaction in the cytoplasm and mucus granules of the epithelial cells of the foveolus and of the mucus layer.
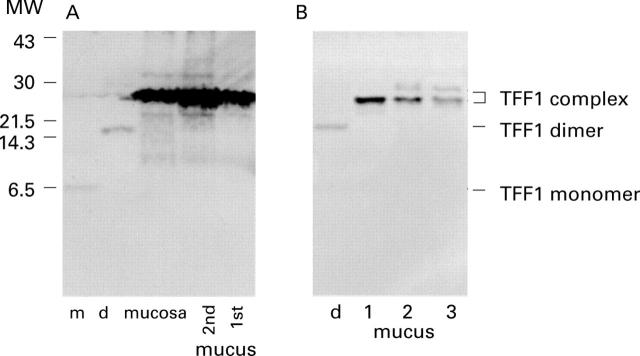
Figure 6 .
TFF1 complex in adherent gastric mucus. Adherent gastric mucus (1st mucus), residual mucus (2nd mucus), and the underlying mucosal layer (mucosa) were obtained from the antrum of a gastrectomy specimen. (A) The proteins were solubilised with non-reducing gel electrophoresis sample buffer. (B) Gastric mucus was obtained from antral brushings taken from three individuals at endoscopy by suspension in inhibitor buffer. Each brushing was suspended in 200 µl and 2 µl aliquots were electrophoresed. Proteins were separated on 20% polyacrylamide gels, transferred to PVDF membrane, and reacted with anti-TFF1 antisera. The resultant membranes are shown (A and B) with the positions of the TFF1 complexes, TFF1 dimer, and TFF1 monomer indicated on the right.
Figure 7 .
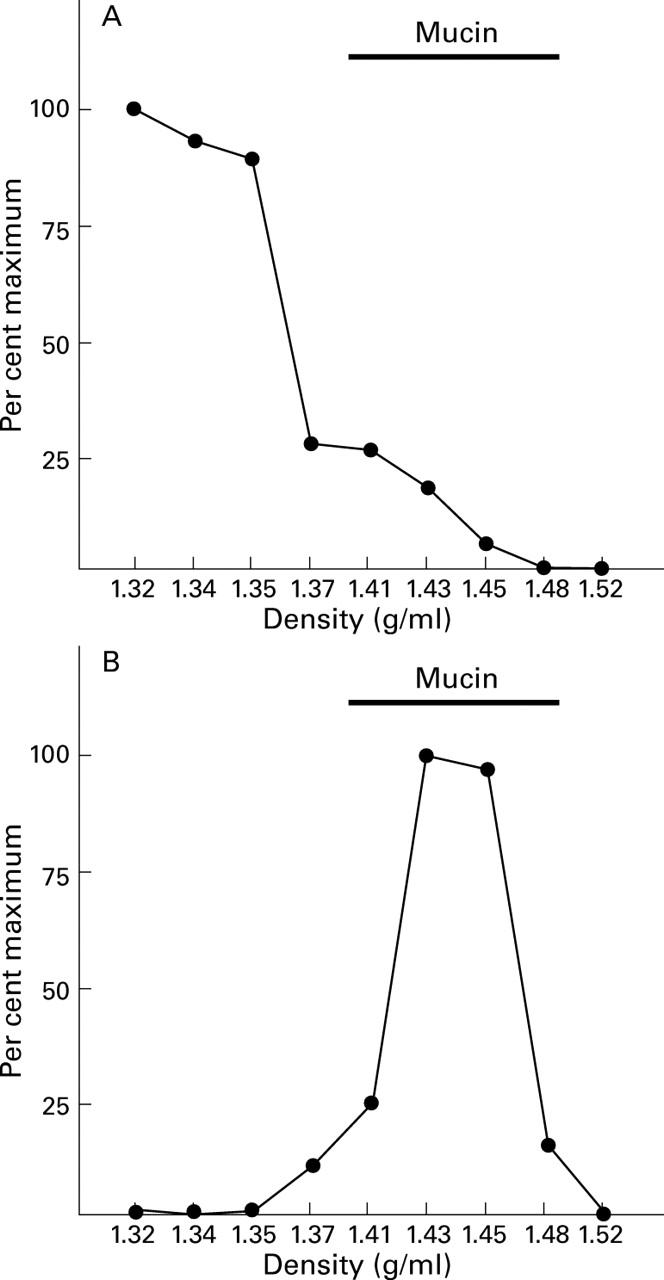
Association of different molecular forms of TFF1 with adherent gastric mucus. Adherent gastric mucus was obtained from five patients at routine endoscopy, dispersed in inhibitor buffer, and caesium chloride was added to a final concentration of 1.42 g/ml. After centrifugation to equilibrium, the gradient was divided into nine equal fractions which were analysed for the presence of TFF1 under non-reducing conditions by western transfer. The intensity of the reaction with the different protein bands was quantified by image analysis and expressed as a percentage of the maximum intensity for either the TFF1 complex (A) or the TFF1 dimer (B).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Allen A., Flemström G., Garner A., Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993 Oct;73(4):823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- Babyatsky M. W., deBeaumont M., Thim L., Podolsky D. K. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996 Feb;110(2):489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Carr M. D., Bauer C. J., Gradwell M. J., Feeney J. Solution structure of a trefoil-motif-containing cell growth factor, porcine spasmolytic protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2206–2210. doi: 10.1073/pnas.91.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M. P., May F. E., Westley B. R. Production and comparison of mature single-domain 'trefoil' peptides pNR-2/pS2 Cys58 and pNR-2/pS2 Ser58. Biochem J. 1995 Jun 15;308(Pt 3):1001–1007. doi: 10.1042/bj3081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M. P., Westley B. R., May F. E. Homodimerization and hetero-oligomerization of the single-domain trefoil protein pNR-2/pS2 through cysteine 58. Biochem J. 1997 Oct 1;327(Pt 1):117–123. doi: 10.1042/bj3270117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajhede M., Petersen T. N., Henriksen A., Petersen J. F., Dauter Z., Wilson K. S., Thim L. Pancreatic spasmolytic polypeptide: first three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure. 1993 Dec 15;1(4):253–262. doi: 10.1016/0969-2126(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Hauser F., Hoffmann W. P-domains as shuffled cysteine-rich modules in integumentary mucin C.1 (FIM-C.1) from Xenopus laevis. Polydispersity and genetic polymorphism. J Biol Chem. 1992 Dec 5;267(34):24620–24624. [PubMed] [Google Scholar]
- Hauser F., Poulsom R., Chinery R., Rogers L. A., Hanby A. M., Wright N. A., Hoffmann W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Tomita M., Uchino H., Kobayashi T., Kataoka H., Sekiya R., Nawa Y. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996 Sep 15;318(Pt 3):939–944. doi: 10.1042/bj3180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey G. P., Oates P. S., Wang T. C., Babyatsky M. W., Brand S. J. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994 Feb;106(2):336–345. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- Jordan N., Newton J., Pearson J., Allen A. A novel method for the visualization of the in situ mucus layer in rat and man. Clin Sci (Lond) 1998 Jul;95(1):97–106. [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Chenard M. P., Masson R., Linares J., Dierich A., LeMeur M., Wendling C., Tomasetto C., Chambon P., Rio M. C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996 Oct 11;274(5285):259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Kédinger M., Chenard M. P., Tomasetto C., Chambon P., Rio M. C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993 Jul;122(1):191–198. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Marchbank T., Westley B. R., May F. E., Calnan D. P., Playford R. J. Dimerization of human pS2 (TFF1) plays a key role in its protective/healing effects. J Pathol. 1998 Jun;185(2):153–158. doi: 10.1002/(SICI)1096-9896(199806)185:2<153::AID-PATH87>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mashimo H., Wu D. C., Podolsky D. K., Fishman M. C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996 Oct 11;274(5285):262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Trefoil proteins: their role in normal and malignant cells. J Pathol. 1997 Sep;183(1):4–7. doi: 10.1002/(SICI)1096-9896(199709)183:1<4::AID-PATH1099>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Newton J. L., Jordan N., Oliver L., Strugala V., Pearson J., James O. F., Allen A. Helicobacter pylori in vivo causes structural changes in the adherent gastric mucus layer but barrier thickness is not compromised. Gut. 1998 Oct;43(4):470–475. doi: 10.1136/gut.43.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Goodlad R. A., Chinery R. A., Poulsom R., Hanby A. M. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2137–2142. doi: 10.1073/pnas.93.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Polshakov V. I., Williams M. A., Gargaro A. R., Frenkiel T. A., Westley B. R., Chadwick M. P., May F. E., Feeney J. High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein. J Mol Biol. 1997 Mar 28;267(2):418–432. doi: 10.1006/jmbi.1997.0896. [DOI] [PubMed] [Google Scholar]
- Poulsom R. Trefoil peptides. Baillieres Clin Gastroenterol. 1996 Mar;10(1):113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Ribieras S., Tomasetto C., Rio M. C. The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim Biophys Acta. 1998 Aug 19;1378(1):F61–F77. doi: 10.1016/s0304-419x(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Lepage P., Diemunsch P., Roitsch C., Chambon P. Structure primaire de la protéine humaine pS2. C R Acad Sci III. 1988;307(19):825–831. [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydr Res. 1988 Jul 15;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Podolsky D. K., Engel E., Guth P. H., Kaunitz J. D. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997 Jun;272(6 Pt 1):G1473–G1480. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]