Abstract
Ciprofloxacin accumulation in Pseudomonas aeruginosa was measured by a bioassay. Drug accumulation in strain PAO2 was compared with that of three spontaneous ciprofloxacin-resistant mutants selected with 0.5 micrograms of ciprofloxacin per ml. PAO4701 cfxA2 contains a mutation in the gyrA gene, PAO4742 cfxB5 may represent a permeability mutant based on pleiotropic drug resistance, and PAO4700 cfxA1 cfxB1 contains both types of mutations. In all strains, drug accumulation was similar, reaching steady state during the first minute of exposure. Drug accumulation was unsaturable over a range of 5 to 80 micrograms/ml, suggesting that ciprofloxacin accumulates by diffusion in P. aeruginosa. Although all four strains accumulated two- to sevenfold more ciprofloxacin in the presence of the inhibitor carbonyl cyanide m-chlorophenylhydrazone, the cfxB mutants accumulated two- to fourfold less drug than either PAO2 or the cfxA2 mutant. Polyacrylamide gel analysis revealed a protein common to cfxB mutants only, while all strains had similar lipopolysaccharide profiles. The results suggest that ciprofloxacin accumulation in P. aeruginosa is a complex phenomenon that may be affected by both an energy-dependent drug efflux process and outer envelope composition.
Full text
PDF

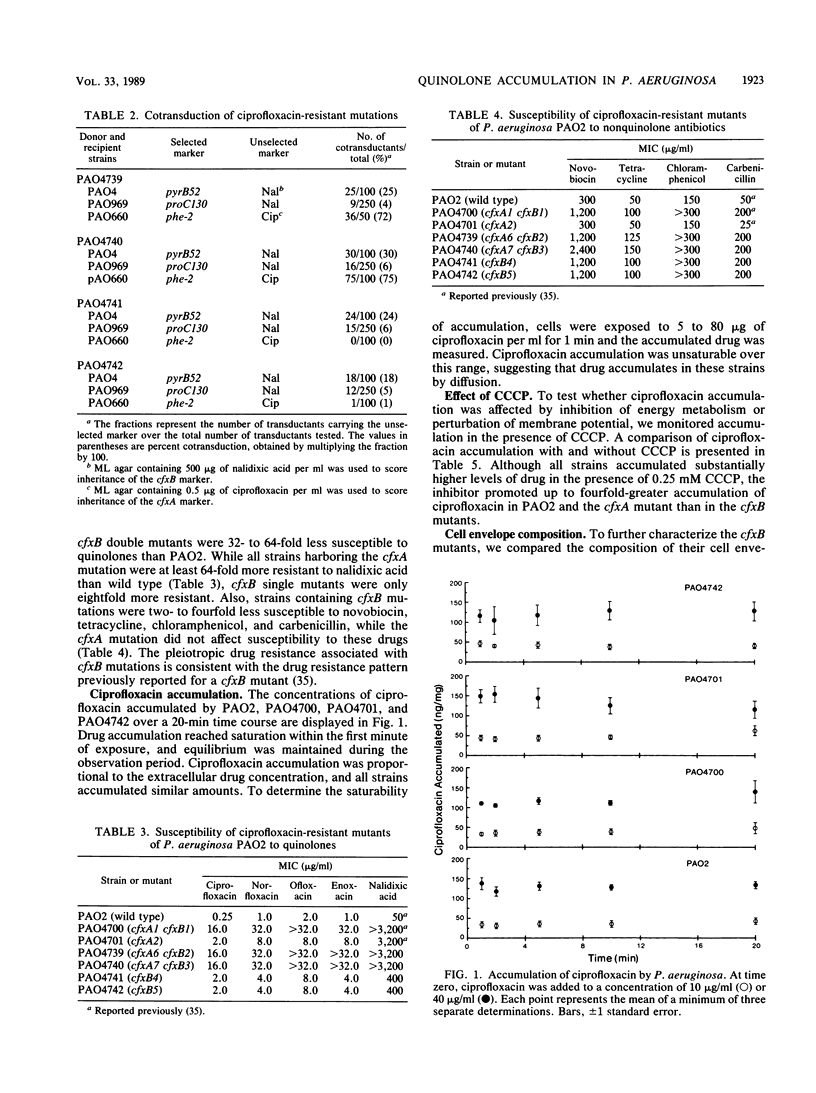
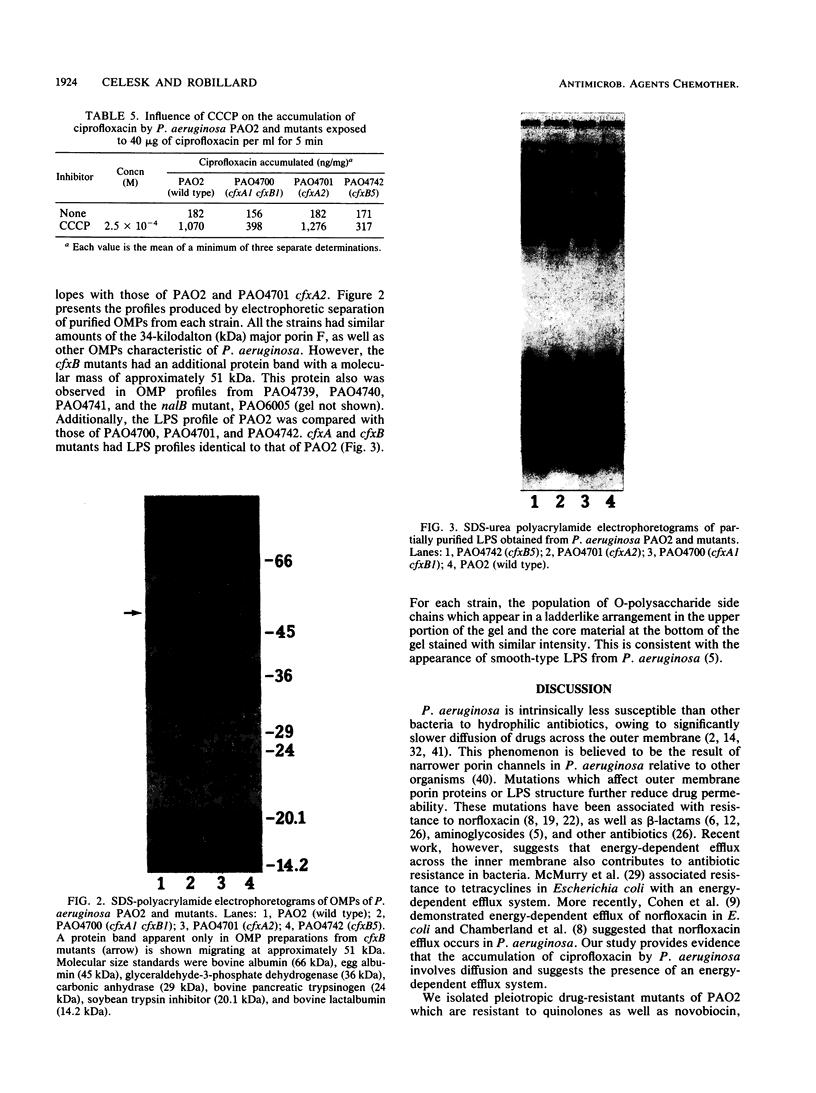


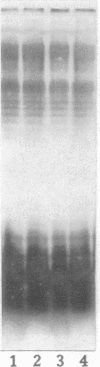
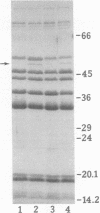
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Fujii T., Fujimaki K., Inoue M., Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition by quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Feary T. W. Transductional analysis of Pseudomonas aeruginosa methionineless auxotrophs. J Bacteriol. 1969 Jan;97(1):210–216. doi: 10.1128/jb.97.1.210-216.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feary T. W., Williams B., Calhoun D. H., Walker T. A. An analysis of arginine requiring mutants in Pseudomonas aeruginosa. Genetics. 1969 Jul;62(3):673–686. [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Moranchel A. H., Duran S., Pichardo A., Magana J. L., Painter B., Forrest A., Drusano G. L. Multiple-dose pharmacokinetics of ciprofloxacin administered intravenously to normal volunteers. Antimicrob Agents Chemother. 1985 Aug;28(2):235–239. doi: 10.1128/aac.28.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Intrinsic antibiotic resistance of Pseudomonas aeruginosa. J Antimicrob Chemother. 1986 Dec;18(6):653–656. doi: 10.1093/jac/18.6.653. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Aronson D. A., Levy S. B. Susceptible Escherichia coli cells can actively excrete tetracyclines. Antimicrob Agents Chemother. 1983 Oct;24(4):544–551. doi: 10.1128/aac.24.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. Comparisons of F factors and R factors: existence of independent regulation groups in F factors. J Bacteriol. 1970 Jul;103(1):81–88. doi: 10.1128/jb.103.1.81-88.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Feary T. W., Blevins W. T. Pyruvate carboxylase deficiency in pleiotropic carbohydrate-negative mutant strains of Pseudomonas aeruginosa. J Bacteriol. 1974 Jun;118(3):999–1009. doi: 10.1128/jb.118.3.999-1009.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Phibbs P. V., Jr Characterization and genetic mapping of fructose phosphotransferase mutations in Pseudomonas aeruginosa. J Bacteriol. 1982 Mar;149(3):897–905. doi: 10.1128/jb.149.3.897-905.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]