Abstract
BACKGROUND—Sodium butyrate, a product of colonic bacterial fermentation, is able to inhibit cell proliferation and to stimulate cell differentiation of colonic epithelial cell lines. It has been proposed that these cellular effects could be linked to its ability to cause hyperacetylation of histone through the inhibition of histone deacetylase. AIM—To analyse the molecular mechanisms of butyrate action on cell proliferation/differentiation and to compare them with those of trichostatin A, a well known inhibitor of histone deacetylase. METHODS—HT-29 cells were grown in the absence or presence of butyrate or trichostatin A. Cell proliferation and cell cycle distribution were studied after DNA staining by crystal violet and propidium iodide respectively. Cell cycle regulatory proteins were studied by western blot and reverse transcription-polymerase chain reaction. Cell differentiation was followed by measuring brush border enzyme activities. Histone acetylation was studied by acid/urea/Triton acrylamide gel electrophoresis. RESULTS—Butyrate blocked cells mainly in the G1 phase of the cell cycle, whereas trichostatin A was inhibitory in both G1 and G2 phases. Butyrate inhibited the mRNA expression of cyclin D1 without affecting its protein expression and stimulated the protein expression of cyclin D3 without affecting its mRNA expression. Trichostatin A showed similar effects on cyclin D1 and D3. Butyrate and trichostatin A stimulated p21 expression both at the mRNA and protein levels, whereas their effects on the expression of cyclin dependent kinases were slightly different. Moreover, butyrate strongly stimulated the activity of alkaline phosphatase and dipeptidyl peptidase IV, whereas trichostatin A had no effect. Finally, a six hour exposure to butyrate or trichostatin A induced histone H4 hyperacetylation. At 15 and 24 hours, histone H4 remained hyperacetylated in the presence of butyrate, whereas it returned to control levels in the presence of trichostatin A. CONCLUSIONS—The data may explain how butyrate acts on cell proliferation/differentiation, and they show that trichostatin A does not reproduce every effect of butyrate, mainly because of its shorter half life. Keywords: butyrate; cyclin D; p21; trichostatin A; colonic epithelial cells; histone acetylation
Full Text
The Full Text of this article is available as a PDF (217.7 KB).
Figure 1 .
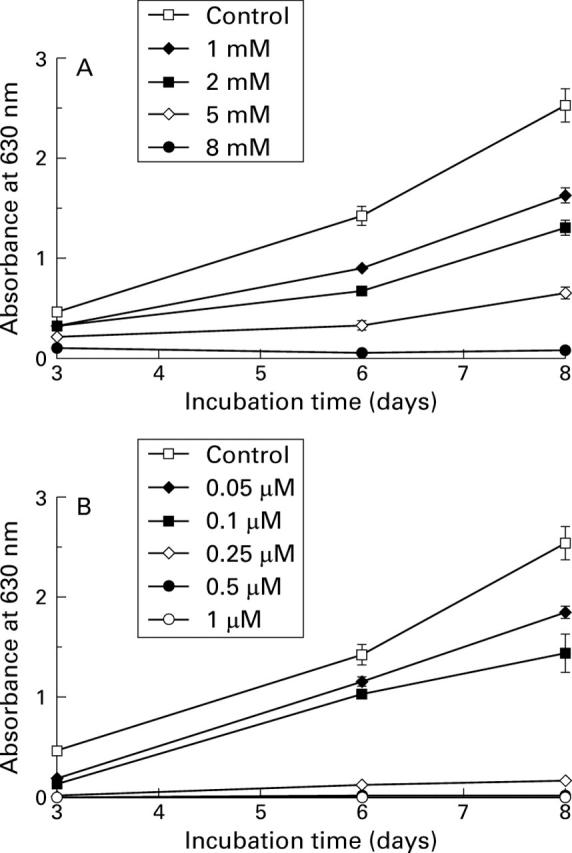
Growth curve of HT-29 cells estimated by the crystal violet staining method. Each point corresponds to the mean and SEM of experiments carried out in quadruplicate. The results presented are from one experiment representative of the four performed. Cells were cultured without or with increasing concentrations of butyrate (A) and trichostatin A (B), as indicated.
Figure 2 .
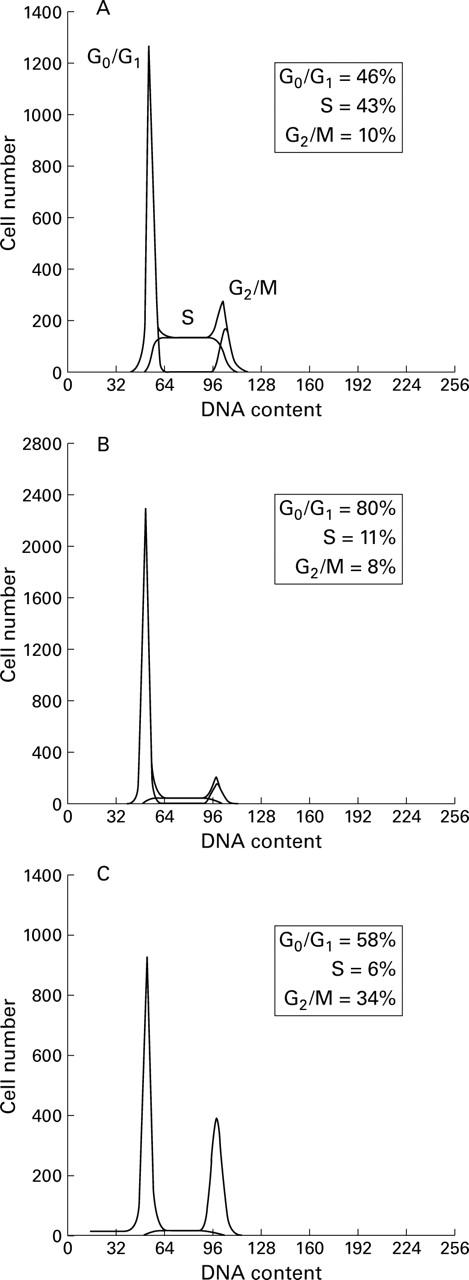
Histograms of DNA content of untreated HT-29 cells (A) and cells treated with 5 mM butyrate (B) or 0.5 µM trichostatin A (C). Cells were treated with each substance for 24 hours, and their DNA content was determined as described in Materials and methods. The cell cycle phase distributions (%) for each treatment are indicated within each panel.
Figure 3 .
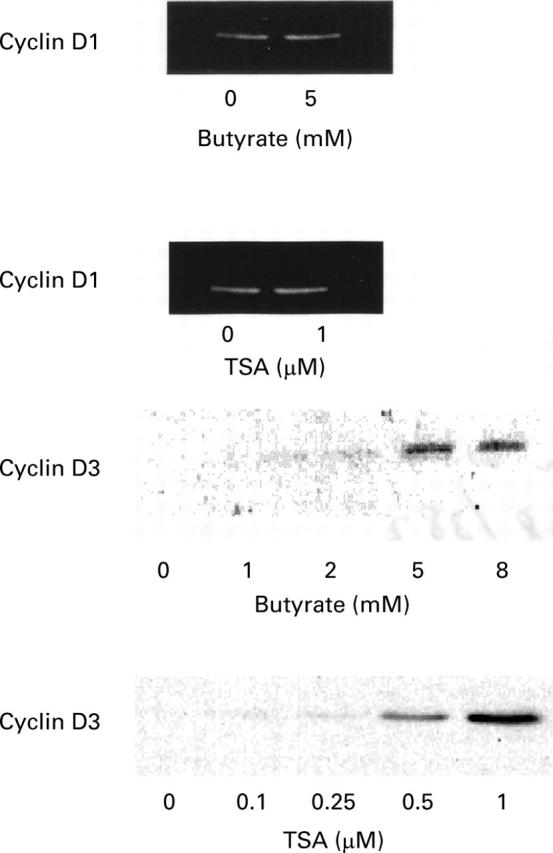
Western blot analysis of cell cycle regulatory protein expression in HT-29 cells treated for 24 hours in the presence of the indicated substances. Equal volumes of whole cell extracts containing 15 µg proteins were separated and electrophoretically blotted. Proteins were probed with antibodies to cyclin D1 and D3, as indicated. TSA, trichostatin A.
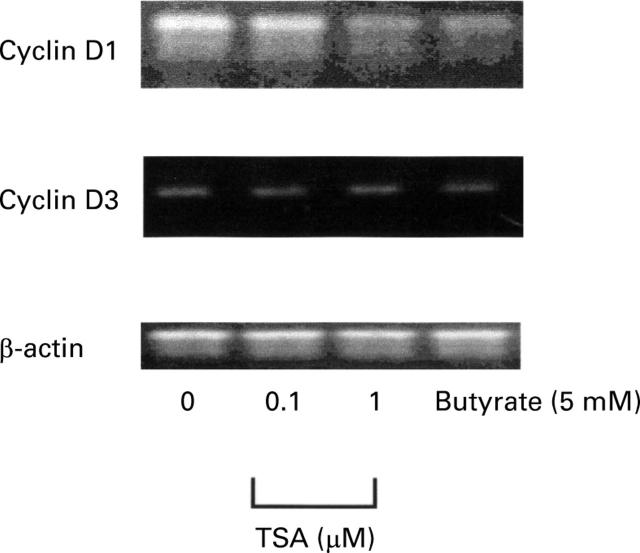
Figure 4 .
Comparison of the effect of trichostatin A (TSA) and butyrate on cyclin D1 and D3 mRNA as studied by reverse transcription-polymerase chain reaction (RT-PCR). PCR products were analysed on a 1.5% agarose gel stained with ethidium bromide. Primers and conditions are specified in Materials and methods. β-Actin is shown as a control. The sizes of the PCR products were 620 bp for β-actin, 577 bp for cyclin D1, and 264 bp for cyclin D3.
Figure 5 .
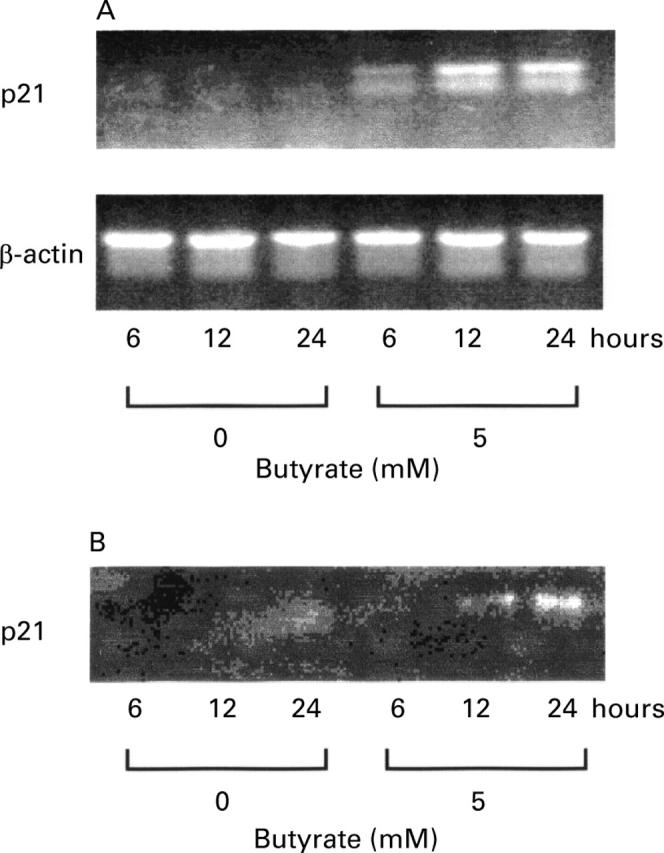
Kinetic analysis of the effect of butyrate on p21 mRNA (A) and protein (B) expression. HT-29 cells were either exposed to medium alone or to 5 mM butyrate, and total mRNA and proteins were extracted at the indicated time after stimulation. In (A), polymerase chain reaction (PCR) products were analysed on a 1.5% agarose gel stained with ethidium bromide. Primers and conditions are specified in Materials and methods. In (B), equal volumes of whole cell extracts containing 15 µg proteins were separated and electrophoretically blotted. Proteins was probed with antibody to p21.
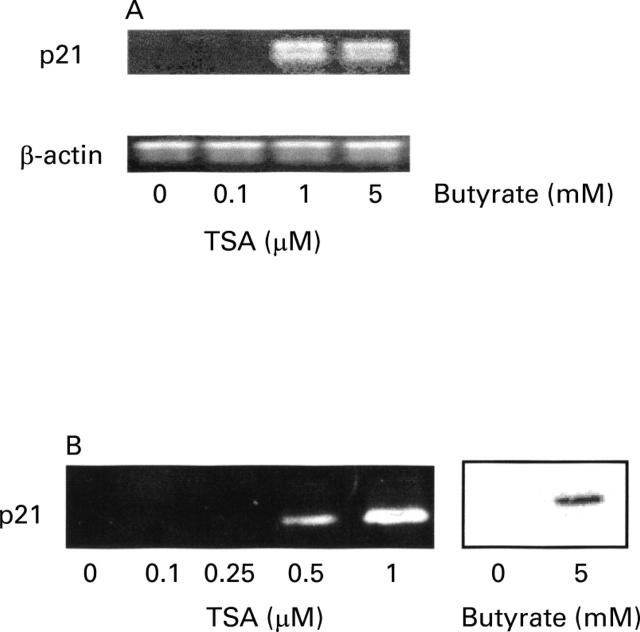
Figure 6 .
Comparison of the effect of trichostatin A (TSA) at increasing concentrations and butyrate (at 5 mM) on the expression of p21 mRNA (A) and protein (B) in HT-29 cells. In (A), polymerase chain reaction (PCR) products were analysed on a 1.5% agarose gel stained with ethidium bromide. Primers and conditions are specified in Materials and methods. The sizes of the PCR products were 620 bp for β-actin and 449 bp for p21. In (B), equal volumes of whole cell extracts containing 15 µg proteins were separated and electrophoretically blotted. Proteins were probed with antibody to p21.
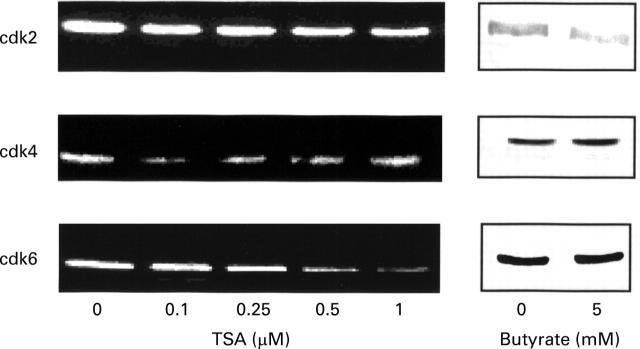
Figure 7 .
Effect of trichostatin A (TSA) and butyrate on the expression of cdk2, cdk4, and cdk6 proteins in HT-29 cells. Equal volumes of whole cell extracts containing 15 µg proteins were separated and electrophoretically blotted. Proteins were probed with antibodies to the indicated proteins.
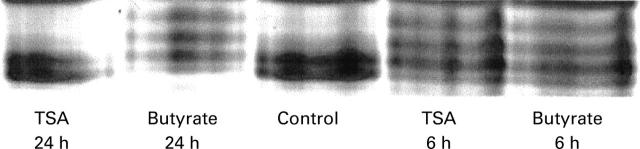
Figure 8 .
Effect of butyrate and trichostatin A (TSA) on histone H4 acetylation. Cells were cultured for six hours and 24 hours in the absence (control) or presence of 5 mM butyrate or 1 µM trichostatin A. Histones were then separated by acid/urea/Triton acrylamide gel electrophoresis, and stained with Coomassie blue.
Figure 9 .
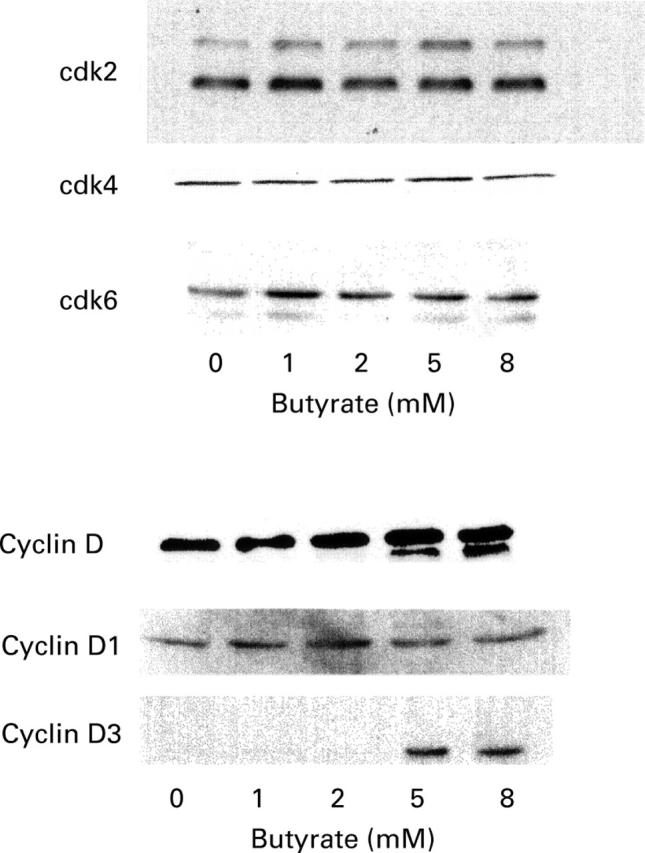
Western blot analysis of cell cycle regulatory protein expression in IEC-6 cells treated for 24 hours in the presence of increasing concentrations of butyrate. Equal volumes of whole cell extracts containing 15 µg proteins were separated and electrophoretically blotted. Proteins were probed with antibodies to cdk2, cdk4, cdk6, cyclins D, D1 and D3, as indicated.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arts J., Lansink M., Grimbergen J., Toet K. H., Kooistra T. Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J. 1995 Aug 15;310(Pt 1):171–176. doi: 10.1042/bj3100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blottière H. M., Zennadi R., Grégoire M., Aillet G., Denis M. G., Meflah K., Le Pendu J. Analysis of the relationship between stage of differentiation and NK/LAK susceptibility of colon carcinoma cells. Int J Cancer. 1993 Feb 1;53(3):409–417. doi: 10.1002/ijc.2910530311. [DOI] [PubMed] [Google Scholar]
- Brasaemle D. L., Attie A. D. Microelisa reader quantitation of fixed, stained, solubilized cells in microtitre dishes. Biotechniques. 1988 May;6(5):418–419. [PubMed] [Google Scholar]
- Böhm S. K., Gum J. R., Jr, Erickson R. H., Hicks J. W., Kim Y. S. Human dipeptidyl peptidase IV gene promoter: tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem J. 1995 Nov 1;311(Pt 3):835–843. doi: 10.1042/bj3110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung Y. S., Song I. S., Erickson R. H., Sleisenger M. H., Kim Y. S. Effect of growth and sodium butyrate on brush border membrane-associated hydrolases in human colorectal cancer cell lines. Cancer Res. 1985 Jul;45(7):2976–2982. [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Csordas A. On the biological role of histone acetylation. Biochem J. 1990 Jan 1;265(1):23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisset L., Tichonicky L., Jaffray P., Delpech M. The effects of sodium butyrate on transcription are mediated through activation of a protein phosphatase. J Biol Chem. 1997 Sep 26;272(39):24148–24153. doi: 10.1074/jbc.272.39.24148. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Xue S. B., Melamed M. R. Effect of n-butyrate on cell cycle progression and in situ chromatin structure of L1210 cells. Exp Cell Res. 1981 Dec;136(2):279–293. doi: 10.1016/0014-4827(81)90006-9. [DOI] [PubMed] [Google Scholar]
- Dion L. D., Goldsmith K. T., Tang D. C., Engler J. A., Yoshida M., Garver R. I., Jr Amplification of recombinant adenoviral transgene products occurs by inhibition of histone deacetylase. Virology. 1997 May 12;231(2):201–209. doi: 10.1006/viro.1997.8538. [DOI] [PubMed] [Google Scholar]
- Gamet L., Daviaud D., Denis-Pouxviel C., Remesy C., Murat J. C. Effects of short-chain fatty acids on growth and differentiation of the human colon-cancer cell line HT29. Int J Cancer. 1992 Sep 9;52(2):286–289. doi: 10.1002/ijc.2910520222. [DOI] [PubMed] [Google Scholar]
- Goldberg Y., Nassif I. I., Pittas A., Tsai L. L., Dynlacht B. D., Rigas B., Shiff S. J. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996 Feb 15;12(4):893–901. [PubMed] [Google Scholar]
- Hague A., Elder D. J., Hicks D. J., Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer. 1995 Jan 27;60(3):400–406. doi: 10.1002/ijc.2910600322. [DOI] [PubMed] [Google Scholar]
- Harb J., Vavasseur F., Chadéneau C., Denis M., Meflah K. Expression of alkaline phosphatase in murine lymphoma cells. Biochem Biophys Res Commun. 1991 May 31;177(1):125–133. doi: 10.1016/0006-291x(91)91957-e. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995 Apr;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerdt B. G., Houston M. A., Augenlicht L. H. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ. 1997 May;8(5):523–532. [PubMed] [Google Scholar]
- Hodin R. A., Meng S., Archer S., Tang R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ. 1996 May;7(5):647–653. [PubMed] [Google Scholar]
- Huang N., Katz J. P., Martin D. R., Wu G. D. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine. 1997 Jan;9(1):27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Collart F. R., Huberman E., Fisher P. B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994 Nov;9(11):3397–3406. [PubMed] [Google Scholar]
- Kiess M., Gill R. M., Hamel P. A. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995 Jan 5;10(1):159–166. [PubMed] [Google Scholar]
- Lallemand F., Courilleau D., Sabbah M., Redeuilh G., Mester J. Direct inhibition of the expression of cyclin D1 gene by sodium butyrate. Biochem Biophys Res Commun. 1996 Dec 4;229(1):163–169. doi: 10.1006/bbrc.1996.1774. [DOI] [PubMed] [Google Scholar]
- Li Y. J., Laurent-Puig P., Salmon R. J., Thomas G., Hamelin R. Polymorphisms and probable lack of mutation in the WAF1-CIP1 gene in colorectal cancer. Oncogene. 1995 Feb 2;10(3):599–601. [PubMed] [Google Scholar]
- McBain J. A., Eastman A., Nobel C. S., Mueller G. C. Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol. 1997 May 9;53(9):1357–1368. doi: 10.1016/s0006-2952(96)00904-5. [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Lilischkis R., Cornish A. L., Lee C. S., Setlur V., Seshadri R., Sutherland R. L. Expression of the cyclin-dependent kinase inhibitors p16INK4, p15INK4B and p21WAF1/CIP1 in human breast cancer. Int J Cancer. 1995 Nov 15;63(4):584–591. doi: 10.1002/ijc.2910630420. [DOI] [PubMed] [Google Scholar]
- Nakano K., Mizuno T., Sowa Y., Orita T., Yoshino T., Okuyama Y., Fujita T., Ohtani-Fujita N., Matsukawa Y., Tokino T. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1997 Aug 29;272(35):22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Park C., Chamberlin M. E., Pan C. J., Chou J. Y. Differential expression and butyrate response of human alkaline phosphatase genes are mediated by upstream DNA elements. Biochemistry. 1996 Jul 30;35(30):9807–9814. doi: 10.1021/bi9602223. [DOI] [PubMed] [Google Scholar]
- Parker M. I., de Haan J. B., Gevers W. DNA hypermethylation in sodium butyrate-treated WI-38 fibroblasts. J Biol Chem. 1986 Feb 25;261(6):2786–2790. [PubMed] [Google Scholar]
- Perrin P., Cassagnau E., Burg C., Patry Y., Vavasseur F., Harb J., Le Pendu J., Douillard J. Y., Galmiche J. P., Bornet F. An interleukin 2/sodium butyrate combination as immunotherapy for rat colon cancer peritoneal carcinomatosis. Gastroenterology. 1994 Dec;107(6):1697–1708. doi: 10.1016/0016-5085(94)90810-9. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Siavoshian S., Blottiere H. M., Cherbut C., Galmiche J. P. Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem Biophys Res Commun. 1997 Mar 6;232(1):169–172. doi: 10.1006/bbrc.1997.6255. [DOI] [PubMed] [Google Scholar]
- Siavoshian S., Blottière H. M., Le Foll E., Kaeffer B., Cherbut C., Galmiche J. P. Comparison of the effect of different short chain fatty acids on the growth and differentiation of human colonic carcinoma cell lines in vitro. Cell Biol Int. 1997 May;21(5):281–287. doi: 10.1006/cbir.1997.0153. [DOI] [PubMed] [Google Scholar]
- Sowa Y., Orita T., Minamikawa S., Nakano K., Mizuno T., Nomura H., Sakai T. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem Biophys Res Commun. 1997 Dec 8;241(1):142–150. doi: 10.1006/bbrc.1997.7786. [DOI] [PubMed] [Google Scholar]
- Steinman R. A., Hoffman B., Iro A., Guillouf C., Liebermann D. A., el-Houseini M. E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994 Nov;9(11):3389–3396. [PubMed] [Google Scholar]
- Taunton J., Hassig C. A., Schreiber S. L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996 Apr 19;272(5260):408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Young G. P., Bhathal P. S. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut. 1986 Dec;27(12):1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Galeazzi D., Schulman H. Acetylation and calcium-dependent phosphorylation of histone H3 in nuclei from butyrate-treated HeLa cells. J Biol Chem. 1983 Jan 25;258(2):1299–1304. [PubMed] [Google Scholar]
- Yasui W., Akama Y., Yokozaki H., Semba S., Kudo Y., Shimamoto F., Tahara E. Expression of p21WAF1/CIP1 in colorectal adenomas and adenocarcinomas and its correlation with p53 protein expression. Pathol Int. 1997 Jul;47(7):470–477. doi: 10.1111/j.1440-1827.1997.tb04526.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Beppu T. Reversible arrest of proliferation of rat 3Y1 fibroblasts in both the G1 and G2 phases by trichostatin A. Exp Cell Res. 1988 Jul;177(1):122–131. doi: 10.1016/0014-4827(88)90030-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kijima M., Akita M., Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990 Oct 5;265(28):17174–17179. [PubMed] [Google Scholar]
- Yoshida M., Nomura S., Beppu T. Effects of trichostatins on differentiation of murine erythroleukemia cells. Cancer Res. 1987 Jul 15;47(14):3688–3691. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]