Abstract
BACKGROUND—TFF2, a member of the trefoil factor family (TFF) of peptides, is a secreted protein of 106 amino acids that is expressed in mucous neck cells of the fundus and glands at the base of the antrum in normal human stomach. TFF2 is also detected at high concentrations around sites of ulceration. It is protective against mucosal damaging agents and stimulates cell motility. AIMS—To measure the expression of TFF2 in normal human stomach and its secretion into gastric juice. METHODS—TFF2 cDNA was amplified by reverse transcription polymerase chain reaction from gastric mucosa and sequenced. Gastric juice or cytosol, prepared from gastric mucosa, was obtained from individuals with macroscopically normal stomachs. TFF2 concentrations were measured by quantitative western transfer analysis. RESULTS—Sequencing of TFF2 cDNA revealed a single amino acid change from the published sequence. Significant amounts of 12 kDa TFF2 were detected in human gastric juice. Larger quantities of a protein of higher apparent molecular mass were also detected. This was shown to be N-glycosylated TFF2 using the endoglycosidase, peptide-N-Gycosidase F. The majority of TFF2 in normal gastric mucosa was also glycosylated. CONCLUSIONS—Human TFF2 is glycosylated via an N-linkage, presumably on Asn15 which forms part of the single consensus site for N-glycosylation in human TFF2. The glycosylation may be of functional significance. Future studies of human TFF2 should use antibodies raised against the correct amino acid sequence. Biological studies should be performed with recombinant protein of the correct sequence, and the biological consequences of glycosylation investigated. Keywords: TFF peptide; stomach; glycosylation; TFF2; restitution
Full Text
The Full Text of this article is available as a PDF (159.3 KB).
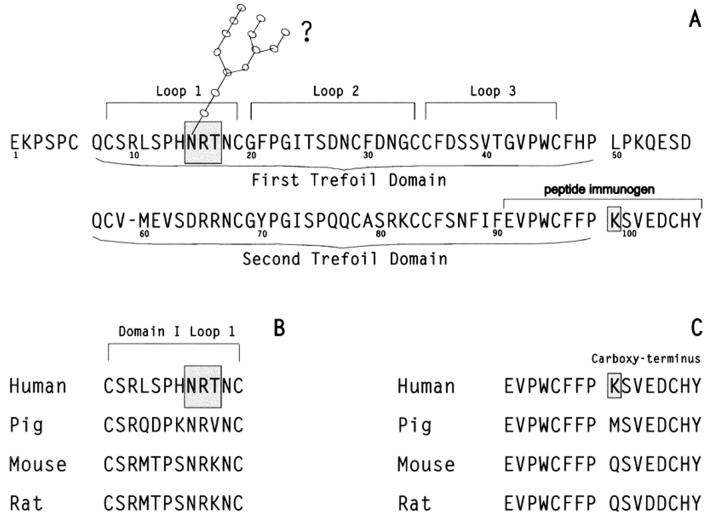
Figure 1 .
Sequence of TFF 2 protein. (A) Sequence of the mature TFF2 protein derived from TFF2 mRNA from human gastric mucosa of three individuals and the TFF2 gene. The positions of the two trefoil domains are indicated as are the amino acids included in each of the three loops formed during folding of the trefoil domains. The single N-glycosylation recognition sequence (NRT) which is a potential glycosylation site located in loop 1 of the first trefoil domain is boxed. The lysine residue (K) which replaces the asparagine residue found in the previously published sequence1 at position 99 is also boxed. The 16 amino acid peptide synthesised for use as an immunogen is overlined. Spaces have been inserted in the sequence after residues 6, 49, and 98 to indicate the limits of the trefoil domains. (B) The amino acid sequences of the first loop in domain I of the four known mammalian TFF2 proteins: human TFF21 (present study); porcine TFF225; murine TFF226; and rat TFF2.27 (C) Comparison of the 16 amino acid residues at the carboxy termini of the four known mammalian TFF2 protein sequences proteins: human TFF2 (present study); porcine TFF225; murine TFF226; and rat TFF2.27
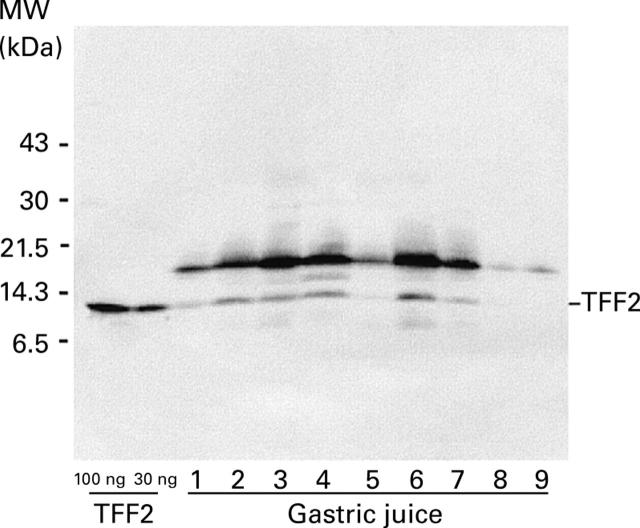
Figure 2 .
Detection of TFF2 in human gastric juice. Aliquots (10 µl) of gastric juice were subjected to electrophoresis on a denaturing polyacrylamide gel, transferred to a PVDF membrane, and reacted with TFF2 antisera. Known amounts of recombinant TFF2 were also analysed. The positions of the molecular mass markers (MW) are shown on the left and of TFF2 on the right hand side.
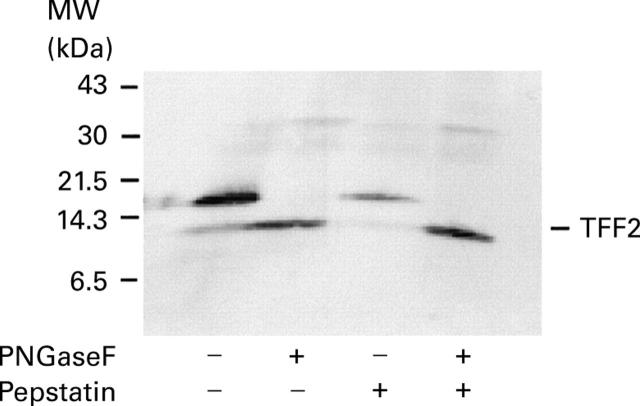
Figure 3 .
Human TFF2 is glycosylated via an N-linkage. Aliquots of gastric juice were denatured and incubated under denaturing conditions in the absence or presence of peptide-N-Gycosidase F (PNGase F), and in the absence or presence of pepstatin. TFF2 was detected by western transfer analysis. The position of TFF2 is shown on the right hand side of the gel and of the molecular weight markers on the left hand side.
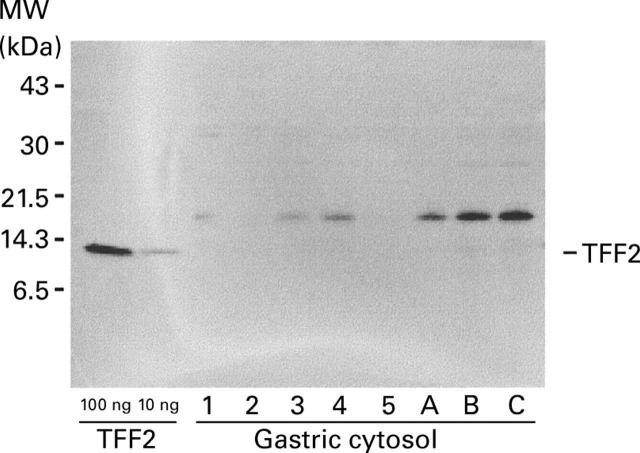
Figure 4 .
Detection of TFF2 in cytosol prepared from human gastric mucosa by western transfer analysis. Cytosol was prepared from small biopsy specimens of normal human gastric mucosa (samples 1-5), or mucosa from gastrectomy specimens (samples A-C). The positions of the molecular mass markers (MW) are shown on the left and of TFF2 on the right hand side.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Babyatsky M. W., deBeaumont M., Thim L., Podolsky D. K. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996 Feb;110(2):489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Carr M. D., Bauer C. J., Gradwell M. J., Feeney J. Solution structure of a trefoil-motif-containing cell growth factor, porcine spasmolytic protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2206–2210. doi: 10.1073/pnas.91.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M. P., Westley B. R., May F. E. Homodimerization and hetero-oligomerization of the single-domain trefoil protein pNR-2/pS2 through cysteine 58. Biochem J. 1997 Oct 1;327(Pt 1):117–123. doi: 10.1042/bj3270117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A., Brown D. G., Gorman M. A., Carr M., Sanderson M. R., Freemont P. S. Crystal structure of a disulfide-linked "trefoil" motif found in a large family of putative growth factors. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1084–1088. doi: 10.1073/pnas.91.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Chinery R., Hanby A. M., Poulsom R., Wright N. A. The production and characterization of a new monoclonal antibody to the trefoil peptide human spasmolytic polypeptide. Histochem J. 1994 Aug;26(8):644–647. doi: 10.1007/BF00158289. [DOI] [PubMed] [Google Scholar]
- Gajhede M., Petersen T. N., Henriksen A., Petersen J. F., Dauter Z., Wilson K. S., Thim L. Pancreatic spasmolytic polypeptide: first three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure. 1993 Dec 15;1(4):253–262. doi: 10.1016/0969-2126(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Jeffrey G. P., Oates P. S., Wang T. C., Babyatsky M. W., Brand S. J. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994 Feb;106(2):336–345. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Kédinger M., Chenard M. P., Tomasetto C., Chambon P., Rio M. C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993 Jul;122(1):191–198. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Cloning of estrogen-regulated messenger RNA sequences from human breast cancer cells. Cancer Res. 1986 Dec;46(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Close physical linkage of the genes encoding the pNR-2/pS2 protein and human spasmolytic protein (hSP). Hum Genet. 1997 Mar;99(3):303–307. doi: 10.1007/s004390050362. [DOI] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Trefoil proteins: their role in normal and malignant cells. J Pathol. 1997 Sep;183(1):4–7. doi: 10.1002/(SICI)1096-9896(199709)183:1<4::AID-PATH1099>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Polshakov V. I., Williams M. A., Gargaro A. R., Frenkiel T. A., Westley B. R., Chadwick M. P., May F. E., Feeney J. High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein. J Mol Biol. 1997 Mar 28;267(2):418–432. doi: 10.1006/jmbi.1997.0896. [DOI] [PubMed] [Google Scholar]
- Poulsom R. Trefoil peptides. Baillieres Clin Gastroenterol. 1996 Mar;10(1):113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Seib T., Blin N., Hilgert K., Seifert M., Theisinger B., Engel M., Dooley S., Zang K. D., Welter C. The three human trefoil genes TFF1, TFF2, and TFF3 are located within a region of 55 kb on chromosome 21q22.3. Genomics. 1997 Feb 15;40(1):200–202. doi: 10.1006/geno.1996.4511. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thim L., Norris K., Norris F., Nielsen P. F., Bjørn S. E., Christensen M., Petersen J. Purification and characterization of the trefoil peptide human spasmolytic polypeptide (hSP) produced in yeast. FEBS Lett. 1993 Mar 8;318(3):345–352. doi: 10.1016/0014-5793(93)80543-4. [DOI] [PubMed] [Google Scholar]
- Thim L., Thomsen J., Christensen M., Jørgensen K. H. The amino acid sequence of pancreatic spasmolytic polypeptide. Biochim Biophys Acta. 1985 Mar 1;827(3):410–418. doi: 10.1016/0167-4838(85)90226-2. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997 Dec;53(11-12):888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., Knowland J. An estrogen receptor from Xenopus laevis liver possibly connected with vitellogenin synthesis. Cell. 1978 Oct;15(2):367–374. doi: 10.1016/0092-8674(78)90005-3. [DOI] [PubMed] [Google Scholar]