Abstract
BACKGROUND—Excessive mucosal generation of cytokines and eicosanoids has been reported in vitro in ulcerative colitis (UC) using traumatising biopsy techniques, and in vivo using time consuming rectal dialysis. AIMS—To validate a simple filter paper technique to profile rectal mucosal production of cytokines and eicosanoids in vivo in patients with UC compared with controls. PATIENTS—Forty one patients with UC (21 with active disease) and 16 controls were studied. METHODS—In vitro, recovery of known concentrations of cytokine or mediator applied to filter papers was measured by ELISA following incubation in buffer. In vivo, patients and controls had filter papers apposed to the rectal mucosa briefly through a rigid sigmoidoscope. Filter papers were then incubated prior to assay by ELISA. RESULTS—In vitro validation studies showed that the filter paper technique could be used to measure mucosal release of interleukin-1β (IL-1β), tumour necrosis factor α (TNF-α), thromboxane B2 (TXB2), and prostaglandin E2 (PGE2), but not interferon γ (IFN-γ). Mucosal release of IL-1β, TNF-α, TXB2 and PGE2 were significantly increased in active UC (p=0.001) and correlated directly with disease activity (p=0.02). CONCLUSIONS—The filter paper technique confirmed increased rectal mucosal release of cytokines and eicosanoids in UC, in proportion to disease activity. The simplicity, safety and speed of the technique make it a practicable option for use in the outpatient clinic to study the pathogenesis of inflammatory bowel disease, and potentially its response to treatment. Keywords: cytokines; eicosanoids; ulcerative colitis; rectal dialysis
Full Text
The Full Text of this article is available as a PDF (172.2 KB).
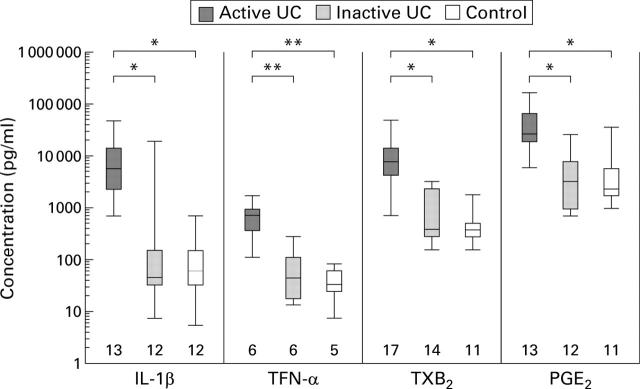
Figure 1 .
Rectal mucosal release of interleukin 1β (IL-β), tumour necrosis factor α (TNF-α), thromboxane B2 (TXB2), and prostaglandin E2 (PGE2), measured by the filter paper technique in active ulcerative colitis (UC), inactive UC (defined by sigmoidoscopic score24) and in controls. Box and whisker plots are shown where the box is the interquartile range, the horizontal line the median, and the whiskers show the highest and lowest values. Numbers of patients studied are shown above the x axis. *p<0.0001, **p<0.001.
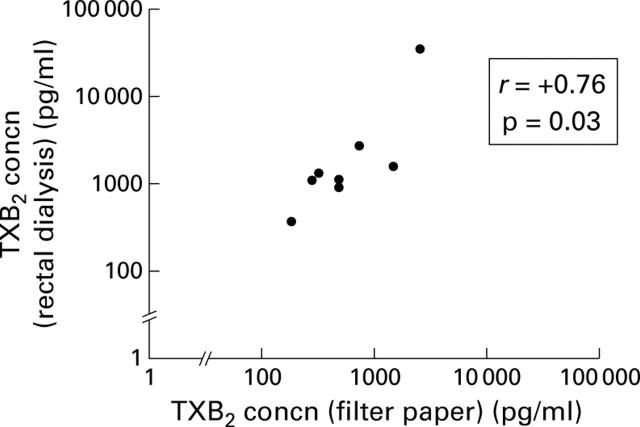
Figure 2 .
Correlation of measurements of rectal mucosal thromboxane (TXB2) release using the filter paper method and rectal dialysis. Two patients with active ulcerative colitis underwent the filter paper technique and two hour rectal dialysis on eight occasions. The x axis shows the values obtained by the filter paper method and the y axis the values obtained by rectal dialysis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON J. H., CONNELL A. M., LENNARD-JONES J. E. VARIATION BETWEEN OBSERVERS IN DESCRIBING MUCOSAL APPEARANCES IN PROCTOCOLITIS. Br Med J. 1964 Jan 11;1(5375):89–92. doi: 10.1136/bmj.1.5375.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Braegger C. P., Nicholls S., Murch S. H., Stephens S., MacDonald T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992 Jan 11;339(8785):89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Cappello M., Keshav S., Prince C., Jewell D. P., Gordon S. Detection of mRNAs for macrophage products in inflammatory bowel disease by in situ hybridisation. Gut. 1992 Sep;33(9):1214–1219. doi: 10.1136/gut.33.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas F., Borruel N., Papo M., Antolín M., Armengol J. R., Malagelada J. R. Usefulness of rectal dialysis to determine intrarectal eicosanoids release in ulcerative colitis. Rev Esp Enferm Dig. 1997 Apr;89(4):280–288. [PubMed] [Google Scholar]
- Casellas F., Borruel N., Papo M., Guarner F., Antolín M., Videla S., Malagelada J. R. Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis. 1998 Feb;4(1):1–5. doi: 10.1097/00054725-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Casellas F., Papo M., Guarner F., Antolín M., Armengol J. R., Malagelada J. R. Intraluminal colonic release of immunoreactive tumour necrosis factor in chronic ulcerative colitis. Clin Sci (Lond) 1994 Oct;87(4):453–458. doi: 10.1042/cs0870453. [DOI] [PubMed] [Google Scholar]
- Casellas F., Papo M., Guarner F., Antolín M., Segura R. M., Armengol J. R., Malagelada J. R. Effects of thromboxane synthase inhibition on in vivo release of inflammatory mediators in chronic ulcerative colitis. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):221–226. [PubMed] [Google Scholar]
- Collins C. E., Benson M. J., Burnham W. R., Rampton D. S. Picotamide inhibition of excess in vitro thromboxane B2 release by colorectal mucosa in inflammatory bowel disease. Aliment Pharmacol Ther. 1996 Jun;10(3):315–320. doi: 10.1111/j.0953-0673.1996.00315.x. [DOI] [PubMed] [Google Scholar]
- Daig R., Andus T., Aschenbrenner E., Falk W., Schölmerich J., Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996 Feb;38(2):216–222. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne S., Hiscott J., D'Agata I., Duhaime A., Seidman E. G. Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci. 1997 Jul;42(7):1557–1566. doi: 10.1023/a:1018895500721. [DOI] [PubMed] [Google Scholar]
- Gertner D. J., Rampton D. S., Stevens T. R., Lennard-Jones J. E. Verapamil inhibits in-vitro leucotriene B4 release by rectal mucosa in active ulcerative colitis. Aliment Pharmacol Ther. 1992 Apr;6(2):163–168. doi: 10.1111/j.1365-2036.1992.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Goodman M. J., Skinner J. M., Truelove S. C. Abnormalities in the apparently normal bowel mucosa in Crohn's disease. Lancet. 1976 Feb 7;1(7954):275–278. doi: 10.1016/s0140-6736(76)91404-5. [DOI] [PubMed] [Google Scholar]
- Hawkey C. J., Karmeli F., Rachmilewitz D. Imbalance of prostacyclin and thromboxane synthesis in Crohn's disease. Gut. 1983 Oct;24(10):881–885. doi: 10.1136/gut.24.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel J., Nielsen O. H., Madsen S., Brynskov J. A simple filter-paper technique allows detection of mucosal cytokine levels in vivo in ulcerative colitis. Interleukin-1 and interleukin-1-receptor antagonist. Dig Dis Sci. 1996 Sep;41(9):1775–1779. doi: 10.1007/BF02088744. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Korelitz B. I., Sommers S. C. Rectal biopsy in patients with Crohn's disease. Normal mucosa on sigmoidoscopic examination. JAMA. 1977 Jun 20;237(25):2742–2744. [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. In vivo profiles of eicosanoids in ulcerative colitis, Crohn's colitis, and Clostridium difficile colitis. Gastroenterology. 1988 Jul;95(1):11–17. doi: 10.1016/0016-5085(88)90284-3. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Lamming C. E., Gallagher A., Hawthorne A. B., Hawkey C. J. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991 Jan;32(1):50–54. doi: 10.1136/gut.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Iwai S., Tanaka T., Hayakawa S. Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J Clin Lab Immunol. 1995;46(3):111–123. [PubMed] [Google Scholar]
- Nielsen O. H., Rask-Madsen J. Mediators of inflammation in chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1996;216:149–159. doi: 10.3109/00365529609094569. [DOI] [PubMed] [Google Scholar]
- O'Morain C., Bishop A. E., McGregor G. P., Levi A. J., Bloom S. R., Polak J. M., Peters T. J. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984 Jan;25(1):57–61. doi: 10.1136/gut.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S., Farr D. H., Goodson J. M. Measurement of prostaglandin E in crevicular fluid. J Clin Periodontol. 1981 Aug;8(4):359–367. doi: 10.1111/j.1600-051x.1981.tb02045.x. [DOI] [PubMed] [Google Scholar]
- Powell-Tuck J., Day D. W., Buckell N. A., Wadsworth J., Lennard-Jones J. E. Correlations between defined sigmoidoscopic appearances and other measures of disease activity in ulcerative colitis. Dig Dis Sci. 1982 Jun;27(6):533–537. doi: 10.1007/BF01296733. [DOI] [PubMed] [Google Scholar]
- Raab Y., Gerdin B., Ahlstedt S., Hällgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993 Sep;34(9):1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab Y., Hällgren R., Knutson L., Krog M., Gerdin B. A technique for segmental rectal and colonic perfusion in humans. Am J Gastroenterol. 1992 Oct;87(10):1453–1459. [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Eliakim R. Platelet-activating factor--a possible mediator in the pathogenesis of ulcerative colitis. Scand J Gastroenterol Suppl. 1990;172:19–21. doi: 10.3109/00365529009091904. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Collins C. E. Review article: thromboxanes in inflammatory bowel disease--pathogenic and therapeutic implications. Aliment Pharmacol Ther. 1993 Aug;7(4):357–367. [PubMed] [Google Scholar]
- Rampton D. S., Hawkey C. J. Prostaglandins and ulcerative colitis. Gut. 1984 Dec;25(12):1399–1413. doi: 10.1136/gut.25.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampton D. S., Murdoch R. D., Sladen G. E. Rectal mucosal histamine release in ulcerative colitis. Clin Sci (Lond) 1980 Nov;59(5):389–391. doi: 10.1042/cs0590389. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Sladen G. E., Youlten L. J. Rectal mucosal prostaglandin E2 release and its relation to disease activity, electrical potential difference, and treatment in ulcerative colitis. Gut. 1980 Jul;21(7):591–596. doi: 10.1136/gut.21.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen J., Grove O., Hansen M. G., Bukhave K., Scient C., Henrik-Nielsen R. Colonic transport of water and electrolytes in a patient with secretory diarrhea due to collagenous colitis. Dig Dis Sci. 1983 Dec;28(12):1141–1146. doi: 10.1007/BF01295815. [DOI] [PubMed] [Google Scholar]
- Reilly A. A., Bellisario R., Pass K. A. Multivariate discrimination for phenylketonuria (PKU) and non-PKU hyperphenylalaninemia after analysis of newborns' dried blood-spot specimens for six amino acids by ion-exchange chromatography. Clin Chem. 1998 Feb;44(2):317–326. [PubMed] [Google Scholar]
- Roberts W. G., Simon T. J., Berlin R. G., Haggitt R. C., Snyder E. S., Stenson W. F., Hanauer S. B., Reagan J. E., Cagliola A., Tanaka W. K. Leukotrienes in ulcerative colitis: results of a multicenter trial of a leukotriene biosynthesis inhibitor, MK-591. Gastroenterology. 1997 Mar;112(3):725–732. doi: 10.1053/gast.1997.v112.pm9041233. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kusugami K., Nishimura H., Ando T., Yamaguchi T., Ohsuga M., Ina K., Enomoto A., Kimura Y., Yoshikai Y. Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology. 1998 Jun;114(6):1237–1243. doi: 10.1016/s0016-5085(98)70430-5. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Hayslett J. P., Binder H. J. Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut. 1986 Mar;27(3):309–316. doi: 10.1136/gut.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J., Hämling J., Koop I., Groessner B., Lochs H., Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999 Feb 6;353(9151):459–461. doi: 10.1016/S0140-6736(98)03339-X. [DOI] [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Shibata M., Takano H., Hironaka T., Hirai K. Detection of human cytomegalovirus DNA in dried newborn blood filter paper. J Virol Methods. 1994 Feb;46(2):279–285. doi: 10.1016/0166-0934(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]