Abstract
BACKGROUND, AIM, AND METHODS—Alpha interferon is the generally approved therapy for HBe antigen positive patients with chronic hepatitis B, but its efficacy is limited. Lamivudine is a new oral nucleoside analogue which potently inhibits hepatitis B virus (HBV) DNA replication. To investigate the possibility of an additive effect of interferon-lamivudine combination therapy compared with interferon or lamivudine monotherapy, we conducted a randomised controlled trial in 230 predominantly Caucasian patients with hepatitis B e antigen (HBeAg) and HBV DNA positive chronic hepatitis B. Previously untreated patients were randomised to receive: combination therapy of lamivudine 100 mg daily with alpha interferon 10 million units three times weekly for 16 weeks after pretreatment with lamivudine for eight weeks (n=75); alpha interferon 10 million units three times weekly for 16 weeks (n=69); or lamivudine 100 mg daily for 52 weeks (n=82). The primary efficacy end point was the HBeAg seroconversion rate at week 52 (loss of HBeAg, development of antibodies to HBeAg and undetectable HBV DNA). RESULTS—The HBeAg seroconversion rate at week 52 was 29% for the combination therapy, 19% for interferon monotherapy, and 18% for lamivudine monotherapy (p=0.12 and p=0.10, respectively, for comparison of the combination therapy with interferon or lamivudine monotherapy). The HBeAg seroconversion rates at week 52 for the combination therapy and lamivudine monotherapy were significantly different in the per protocol analysis (36% (20/56) v 19% (13/70), respectively; p=0.02). The effect of combining lamivudine and interferon appeared to be most useful in patients with moderately elevated alanine aminotransferase levels at baseline. Adverse events with the combination therapy were similar to interferon monotherapy; patients receiving lamivudine monotherapy had significantly fewer adverse events. CONCLUSIONS—HBeAg seroconversion rates at one year were similar for lamivudine monotherapy (52 weeks) and standard alpha interferon therapy (16 weeks). The combination of lamivudine and interferon appeared to increase the HBeAg seroconversion rate, particularly in patients with moderately elevated baseline aminotransferase levels. The potential benefit of combining lamivudine and interferon should be investigated further in studies with different regimens of combination therapy. Keywords: chronic hepatitis B; hepatitis B virus; nucleoside analogue; lamivudine; alpha interferon; combination therapy; HBeAg seroconversion
Full Text
The Full Text of this article is available as a PDF (154.0 KB).
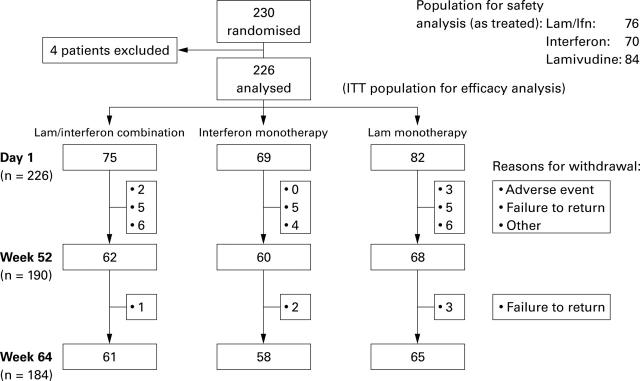
Figure 1 .
Progress of patients through the various stages of the trial.
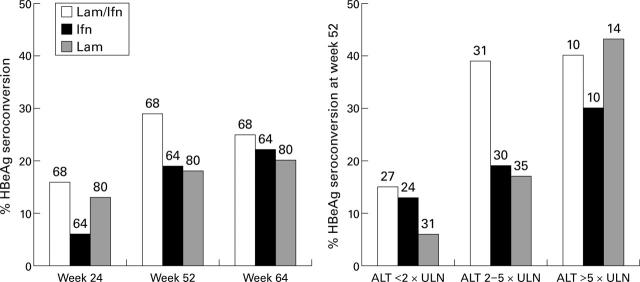
Figure 2 .
Percentage HBeAg seroconversion for the three treatment arms (lamivudine-interferon, interferon, and lamivudine) by various times (left) and by baseline ALT levels at week 52 (right). The number of patients in each category is given above the bar.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brook M. G., Karayiannis P., Thomas H. C. Which patients with chronic hepatitis B virus infection will respond to alpha-interferon therapy? A statistical analysis of predictive factors. Hepatology. 1989 Nov;10(5):761–763. doi: 10.1002/hep.1840100502. [DOI] [PubMed] [Google Scholar]
- Brook M. G., Petrovic L., McDonald J. A., Scheuer P. J., Thomas H. C. Histological improvement after anti-viral treatment for chronic hepatitis B virus infection. J Hepatol. 1989 Mar;8(2):218–225. doi: 10.1016/0168-8278(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Perrillo R. P., Schiff E. R., Bartholomew M., Vicary C., Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995 Dec 21;333(25):1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- Fattovich G., Giustina G., Favarato S., Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996 Jan;24(1):38–47. doi: 10.1016/s0168-8278(96)80184-x. [DOI] [PubMed] [Google Scholar]
- Honkoop P., Niesters H. G., de Man R. A., Osterhaus A. D., Schalm S. W. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997 Jun;26(6):1393–1395. doi: 10.1016/s0168-8278(97)80476-x. [DOI] [PubMed] [Google Scholar]
- Janssen H. L., Gerken G., Carreño V., Marcellin P., Naoumov N. V., Craxi A., Ring-Larsen H., Kitis G., van Hattum J., de Vries R. A. Interferon alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology. 1999 Jul;30(1):238–243. doi: 10.1002/hep.510300113. [DOI] [PubMed] [Google Scholar]
- Knodell R. G., Ishak K. G., Black W. C., Chen T. S., Craig R., Kaplowitz N., Kiernan T. W., Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981 Sep-Oct;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- Krogsgaard K., Bindslev N., Christensen E., Craxi A., Schlichting P., Schalm S., Carreno V., Trepo C., Gerken G., Thomas H. C. The treatment effect of alpha interferon in chronic hepatitis B is independent of pre-treatment variables. Results based on individual patient data from 10 clinical controlled trials. European Concerted Action on Viral Hepatitis (Eurohep). J Hepatol. 1994 Oct;21(4):646–655. doi: 10.1016/s0168-8278(94)80114-2. [DOI] [PubMed] [Google Scholar]
- Lai C. L., Chien R. N., Leung N. W., Chang T. T., Guan R., Tai D. I., Ng K. Y., Wu P. C., Dent J. C., Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998 Jul 9;339(2):61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- Lok A. S., Wu P. C., Lai C. L., Lau J. Y., Leung E. K., Wong L. S., Ma O. C., Lauder I. J., Ng C. P., Chung H. T. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992 Jun;102(6):2091–2097. doi: 10.1016/0016-5085(92)90337-x. [DOI] [PubMed] [Google Scholar]
- Mutimer D., Naoumov N., Honkoop P., Marinos G., Ahmed M., de Man R., McPhillips P., Johnson M., Williams R., Elias E. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998 Jun;28(6):923–929. doi: 10.1016/s0168-8278(98)80338-3. [DOI] [PubMed] [Google Scholar]
- Nevens F., Main J., Honkoop P., Tyrrell D. L., Barber J., Sullivan M. T., Fevery J., De Man R. A., Thomas H. C. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997 Oct;113(4):1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- Niederau C., Heintges T., Lange S., Goldmann G., Niederau C. M., Mohr L., Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996 May 30;334(22):1422–1427. doi: 10.1056/NEJM199605303342202. [DOI] [PubMed] [Google Scholar]
- Perrillo R. P., Schiff E. R., Davis G. L., Bodenheimer H. C., Jr, Lindsay K., Payne J., Dienstag J. L., O'Brien C., Tamburro C., Jacobson I. M. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990 Aug 2;323(5):295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- Renault P. F., Hoofnagle J. H. Side effects of alpha interferon. Semin Liver Dis. 1989 Nov;9(4):273–277. doi: 10.1055/s-2008-1040523. [DOI] [PubMed] [Google Scholar]
- Severini A., Liu X. Y., Wilson J. S., Tyrrell D. L. Mechanism of inhibition of duck hepatitis B virus polymerase by (-)-beta-L-2',3'-dideoxy-3'-thiacytidine. Antimicrob Agents Chemother. 1995 Jul;39(7):1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. K., Cheung A. M., O'Rourke K., Naylor C. D., Detsky A. S., Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993 Aug 15;119(4):312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]
- de Jongh F. E., Janssen H. L., de Man R. A., Hop W. C., Schalm S. W., van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992 Nov;103(5):1630–1635. doi: 10.1016/0016-5085(92)91188-a. [DOI] [PubMed] [Google Scholar]