Abstract
BACKGROUND—Mucosal surface hydrophobicity is a key factor of the gastric acid defence barrier. In the colon, surface hydrophobicity is high but its biological function remains unexplored. AIMS—To investigate the functional changes of the barrier due to removal of the surface active phospholipid layer by a detergent, or to reinforcement of the surface active phospholipid by local application of a suspension of lipids. METHODS—Surface hydrophobicity (contact angle measurement), colonic permeability (lumen to blood clearance of mannitol and dextran), and mucosal resistance against luminal aggression (distal colitis induced by dextran sodium sulphate, DSS) were investigated in three study groups: (a) rats pretreated with a detergent (Brij 35) known to remove surfactant lipids; (b) rats pretreated with a suspension of surface active lipids (tripalmitin and dipalmitoyl-phosphatidylcholine); and (c) control rats pretreated with the corresponding vehicles. RESULTS—In controls, surface hydrophobicity was low on the caecal mucosa and high in colon and rectum. Detergent treatment reduced surface hydrophobicity, and increased colonic permeability to mannitol and dextran. Conversely, treatment with lipids increased surface hydrophobicity, and reduced colonic permeability. Administration of DSS induced a progressive loss of colonic surface hydrophobicity, and an increase in permeability to mannitol and dextran. Detergent treatment increased susceptibility to epithelial damage and mucosal inflammation by DSS. Treatment with lipids reduced susceptibility to DSS colitis. CONCLUSION—Colonic surface hydrophobicity modulates permeability to hydrophilic molecules and protects against toxins. Keywords: mucosal surface hydrophobicity; mannitol; dextran
Full Text
The Full Text of this article is available as a PDF (134.0 KB).
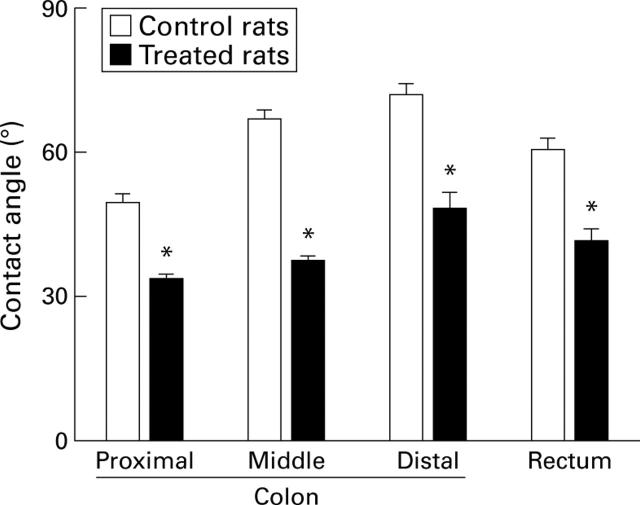
Figure 1 .
Surface hydrophobicity of the colonic mucosa in control rats and in rats treated with 2% Brij 35 in the drinking water for two days. Data are presented as the mean (SEM) of five rats per group. *p<0.05 compared with control.
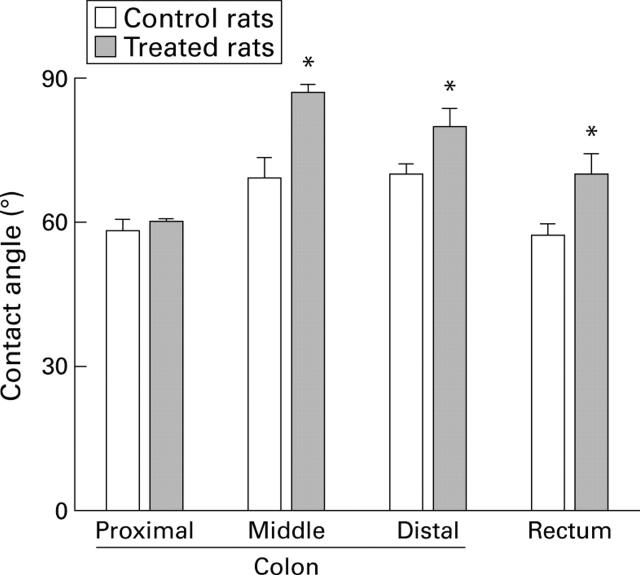
Figure 2 .
Surface hydrophobicity of the colonic mucosa in control rats and in rats treated with 2 ml of tripalmitin-phosphatidylcholine. Data are presented as the mean (SEM) of seven rats per group. *p<0.05 compared with control.
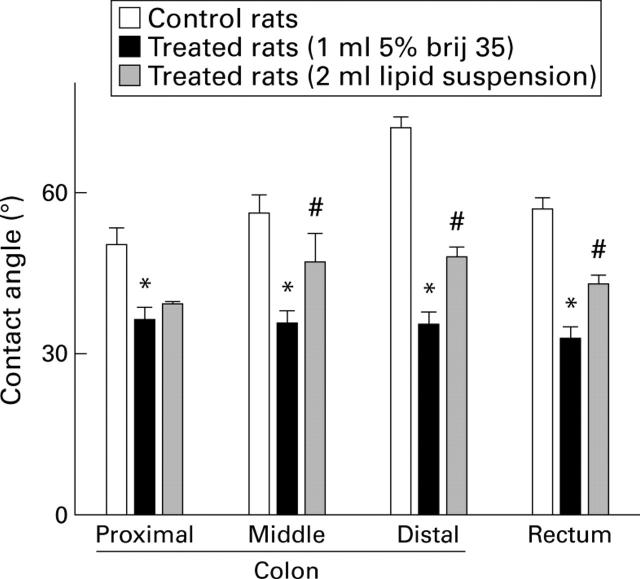
Figure 3 .
Surface hydrophobicity of the colonic mucosa in control rats, in rats treated with 1 ml 5% Brij 35 instilled into the colonic lumen, and in rats treated with 5% Brij 35 as above and 2 ml of a lipid suspension (tripalmitin-phosphatidylcholine) 20 minutes later. Data are presented as the mean (SEM) of five rats per group. *p<0.05 compared with control; #p<0.05 compared with Brij 35 only.
Figure 4 .
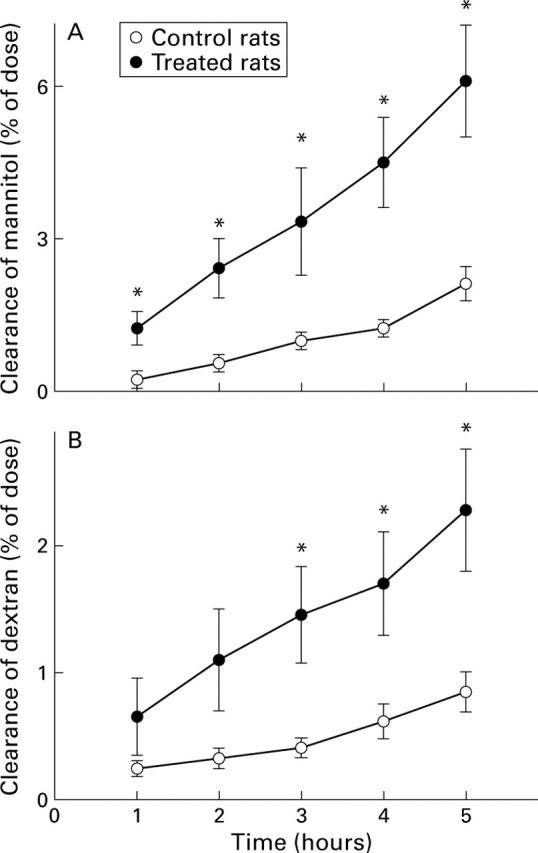
Lumen to blood clearance of mannitol or dextran expressed as percentage of the dose administered intracolonically at time 0 to control rats and to rats treated with 2% Brij 35 in the drinking water for two days. Values are means (SEM) for five rats per group. *p<0.05 compared with control.
Figure 5 .
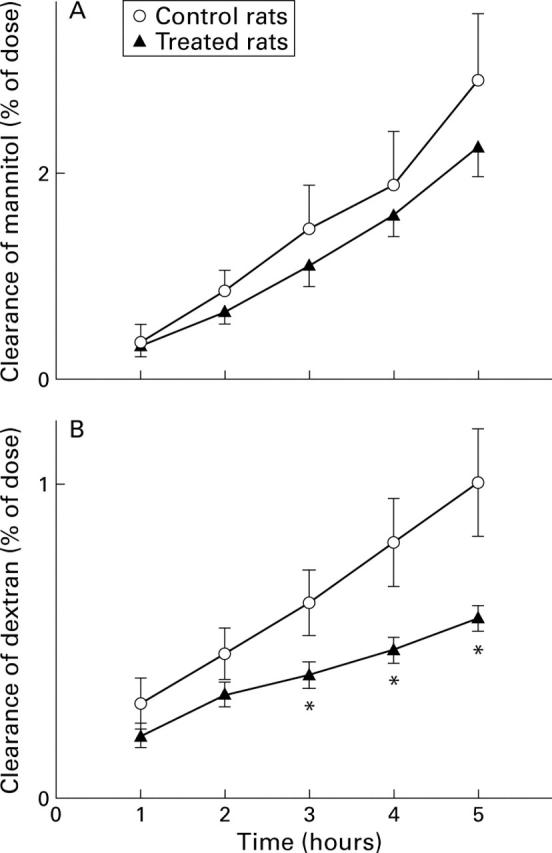
Lumen to blood clearance of mannitol or dextran expressed as percentage of the dose administered intracolonically at time 0 to control rats and to rats treated with 2 ml of a lipid suspension (tripalmitin-phosphatidylcholine) instilled into the colonic lumen. Values are means (SEM) for seven rats per group. *p<0.05 compared with control.
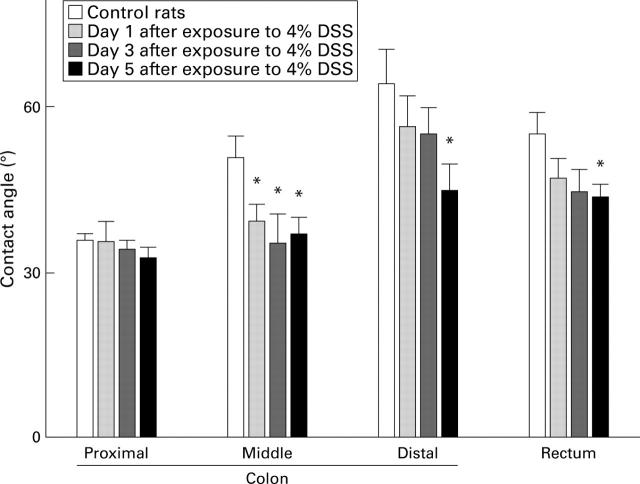
Figure 6 .
Surface hydrophobicity of the colonic mucosa in control rats (day 0) and in rats treated with 4% dextran sodium sulphate (DSS) in the drinking water on days 1, 3, and 5 after exposure to DSS. Values are means (SEM) for five rats per group. *p<0.05 compared with control.
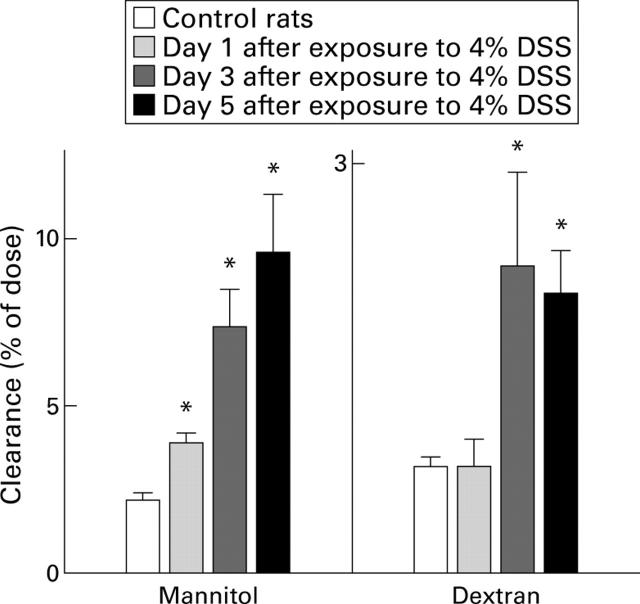
Figure 7 .
Lumen to blood clearance of mannitol or dextran five hours after intracolonic administration of labelled probes in controls, and in rats treated with 4% dextran sodium sulphate (DSS) in the drinking water on days 1, 3, and 5 after exposure to DSS. Values are means (SEM) for 5-8 rats per group. *p<0.05 compared with control.
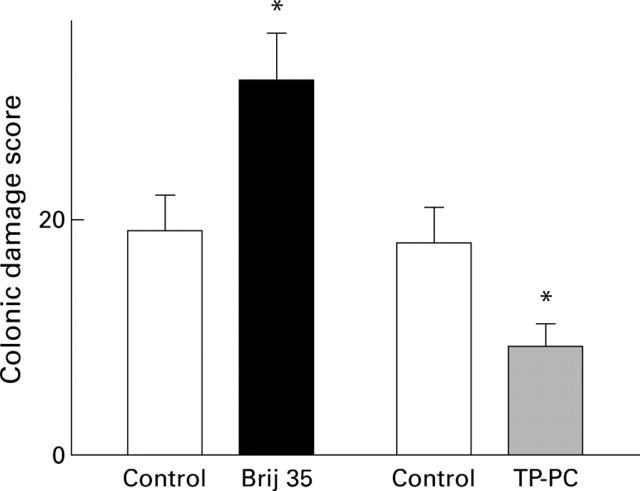
Figure 8 .
Colonic damage scores after administration of 4% DSS for five days in control rats, in rats pretreated with 2% Brij 35 for two days, and in rats treated with 2 ml enemas of a lipid suspension for three days before DSS and during the five days on DSS. Data are presented as the mean (SEM) of five rats per group. *p<0.05 compared with control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler B. D., Lichtenberger L. M., Hills B. A. Distribution of surfactants in the canine gastrointestinal tract and their ability to lubricate. Am J Physiol. 1983 Jun;244(6):G645–G651. doi: 10.1152/ajpgi.1983.244.6.G645. [DOI] [PubMed] [Google Scholar]
- Casellas F., Aguadé S., Soriano B., Accarino A., Molero J., Guarner L. Intestinal permeability to 99mTc-diethylenetriaminopentaacetic acid in inflammatory bowel disease. Am J Gastroenterol. 1986 Sep;81(9):767–770. [PubMed] [Google Scholar]
- Cooper H. S., Murthy S. N., Shah R. S., Sedergran D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993 Aug;69(2):238–249. [PubMed] [Google Scholar]
- Dieleman L. A., Palmen M. J., Akol H., Bloemena E., Peña A. S., Meuwissen S. G., Van Rees E. P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998 Dec;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman L. A., Ridwan B. U., Tennyson G. S., Beagley K. W., Bucy R. P., Elson C. O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994 Dec;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Egger B., Carey H. V., Procaccino F., Chai N. N., Sandgren E. P., Lakshmanan J., Buslon V. S., French S. W., Büchler M. W., Eysselein V. E. Reduced susceptibility of mice overexpressing transforming growth factor alpha to dextran sodium sulphate induced colitis. Gut. 1998 Jul;43(1):64–70. doi: 10.1136/gut.43.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Procaccino F., Lakshmanan J., Reinshagen M., Hoffmann P., Patel A., Reuben W., Gnanakkan S., Liu L., Barajas L. Mice lacking transforming growth factor alpha have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology. 1997 Sep;113(3):825–832. doi: 10.1016/s0016-5085(97)70177-x. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Goetz G. S., Rubio S., Chailley-Heu B., Shao J. S., Ducroc R., Alpers D. H. Isolation and characterization of surfactant-like particles in rat and human colon. Am J Physiol. 1997 Mar;272(3 Pt 1):G425–G434. doi: 10.1152/ajpgi.1997.272.3.G425. [DOI] [PubMed] [Google Scholar]
- Gardiner K. R., Anderson N. H., Rowlands B. J., Barbul A. Colitis and colonic mucosal barrier dysfunction. Gut. 1995 Oct;37(4):530–535. doi: 10.1136/gut.37.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard P. J., Hills B. A., Lichtenberger L. M. Does aspirin damage canine gastric mucosa by reducing its surface hydrophobicity? Am J Physiol. 1987 Mar;252(3 Pt 1):G421–G430. doi: 10.1152/ajpgi.1987.252.3.G421. [DOI] [PubMed] [Google Scholar]
- Goddard P. J., Kao Y. C., Lichtenberger L. M. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990 Feb;98(2):361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- Hills B. A., Butler B. D., Lichtenberger L. M. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983 May;244(5):G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- Hills B. A., Kirwood C. A. Gastric mucosal barrier: barrier to hydrogen ions imparted by gastric surfactant in vitro. Gut. 1992 Aug;33(8):1039–1041. doi: 10.1136/gut.33.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Jenkins R. T., Jones D. B., Goodacre R. L., Collins S. M., Coates G., Hunt R. H., Bienenstock J. Reversibility of increased intestinal permeability to 51Cr-EDTA in patients with gastrointestinal inflammatory diseases. Am J Gastroenterol. 1987 Nov;82(11):1159–1164. [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y. C., Goddard P. J., Lichtenberger L. M. Morphological effects of aspirin and prostaglandin on the canine gastric mucosal surface. Analysis with a phospholipid-selective cytochemical stain. Gastroenterology. 1990 Mar;98(3):592–606. doi: 10.1016/0016-5085(90)90278-9. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Romero J. J., Kao Y. C., Dial E. J. Gastric protective activity of mixtures of saturated polar and neutral lipids in rats. Gastroenterology. 1990 Aug;99(2):311–326. doi: 10.1016/0016-5085(90)91011-t. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- Lugea A., Antolín M., Mourelle M., Guarner F., Malagelada J. R. Deranged hydrophobic barrier of the rat gastroduodenal mucosa after parenteral nonsteroidal anti-inflammatory drugs. Gastroenterology. 1997 Jun;112(6):1931–1939. doi: 10.1053/gast.1997.v112.pm9178685. [DOI] [PubMed] [Google Scholar]
- Lugea A., Mourelle M., Guarner F., Domingo A., Salas A., Malagelada J. R. Phosphatidylcholines as mediators of adaptive cytoprotection of the rat duodenum. Gastroenterology. 1994 Sep;107(3):720–727. doi: 10.1016/0016-5085(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Mack D. R., Neumann A. W., Policova Z., Sherman P. M. Surface hydrophobicity of the intestinal tract. Am J Physiol. 1992 Jan;262(1 Pt 1):G171–G177. doi: 10.1152/ajpgi.1992.262.1.G171. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Ni J., Chen S. F., Hollander D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996 Aug;39(2):234–241. doi: 10.1136/gut.39.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990 Mar;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Procaccino F., Reinshagen M., Hoffmann P., Zeeh J. M., Lakshmanan J., McRoberts J. A., Patel A., French S., Eysselein V. E. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994 Jul;107(1):12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Scheiman J. M., Kraus E. R., Bonnville L. A., Weinhold P. A., Boland C. R. Synthesis and prostaglandin E2-induced secretion of surfactant phospholipid by isolated gastric mucous cells. Gastroenterology. 1991 May;100(5 Pt 1):1232–1240. [PubMed] [Google Scholar]
- Spychal R. T., Marrero J. M., Saverymuttu S. H., Northfield T. C. Measurement of the surface hydrophobicity of human gastrointestinal mucosa. Gastroenterology. 1989 Jul;97(1):104–111. doi: 10.1016/0016-5085(89)91422-4. [DOI] [PubMed] [Google Scholar]
- Stein J., Ries J., Barrett K. E. Disruption of intestinal barrier function associated with experimental colitis: possible role of mast cells. Am J Physiol. 1998 Jan;274(1 Pt 1):G203–G209. doi: 10.1152/ajpgi.1998.274.1.G203. [DOI] [PubMed] [Google Scholar]
- Tatsumi Y., Lichtenberger L. M. Molecular association of trinitrobenzenesulfonic acid and surface phospholipids in the development of colitis in rats. Gastroenterology. 1996 Mar;110(3):780–789. doi: 10.1053/gast.1996.v110.pm8608888. [DOI] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Levi A. J., Menzies I. S., Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992 Mar;33(3):320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner T. G., Cohn S. M., Schloemann S., Stenson W. F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology. 1998 Oct;115(4):874–882. doi: 10.1016/s0016-5085(98)70259-8. [DOI] [PubMed] [Google Scholar]
- Zareie M., McKay D. M., Kovarik G. G., Perdue M. H. Monocyte/macrophages evoke epithelial dysfunction: indirect role of tumor necrosis factor-alpha. Am J Physiol. 1998 Oct;275(4 Pt 1):C932–C939. doi: 10.1152/ajpcell.1998.275.4.C932. [DOI] [PubMed] [Google Scholar]