Abstract
BACKGROUND AND AIMS—The role of altered cell adhesion is critical for the development of epithelial cancers. E-cadherin plays an important role in the maintenance of cell-cell adhesion and its function is thought to be regulated by its associated cytoplasmic proteins, such as α-catenin and β-catenin. To determine the role of α-catenin expression in gastric carcinogenesis, we studied its expression in human gastric cancer and in the gastric mucosa of first degree relatives with no clinical disease. METHODS—α-Catenin expression was assessed by immunohistochemical analysis and reverse transcriptase-polymerase chain reaction (RT-PCR) using gastric tissue specimens from patients with gastric cancer and from the gastric mucosa of first degree relatives of gastric cancer patients and healthy controls. RESULTS—mRNA levels of α-catenin were reduced or absent in 13 of 19 gastric cancer tissues, which differed significantly from levels found in the tumour free gastric mucosa of cancer patients (p<0.05). Of the cancer samples with altered α-catenin mRNA levels, α-catenin expression was negative in seven and decreased in six cases. Interestingly, decreased α-catenin mRNA expression also occurred in the mucosa of the corpus (11/18) and antrum (4/18) of first degree relatives. In the corpus biopsies α-catenin expression was more often decreased or lost compared with the antrum biopsies in first degree relatives and healthy controls (p<0.05). Immunohistochemical analysis revealed membranous expression of α-catenin in gastric cancer cells and the non-malignant gastric epithelium. However, some cancers also exhibited loss of membranous staining. Generally, loss or downregulation of α-catenin mRNA in the gastric mucosa was associated with Helicobacter pylori infection (p<0.05). CONCLUSION—Our findings suggest that loss or downregulation of α-catenin expression may be an early event in gastric carcinogenesis and may be associated with H pylori infection. Keywords: risk; stomach; gene; cadherins; gastric cancer; α-catenin
Full Text
The Full Text of this article is available as a PDF (344.6 KB).
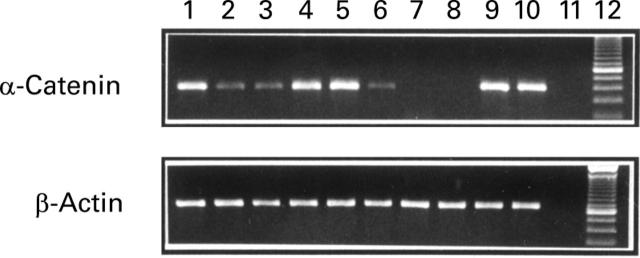
Figure 1 .
RT-PCR analysis of α-catenin mRNA in gastric tissues. RT-PCR analysis was performed using specific primers for human α-catenin as described in materials and methods. Amplification of β-actin mRNA was performed to ensure equal loading. Lanes 1-2: tumour free and tumour from patient No 1; lanes 3-4: corpus and antrum from relative No 6; lanes 6-7: tumour free and tumour from patient No 2; lanes 8-9: corpus and antrum from relative No 2; lanes 5 and 10: gastric mucosa from healthy subjects; lane 11: negative control; and lane 12: DNA ladder.
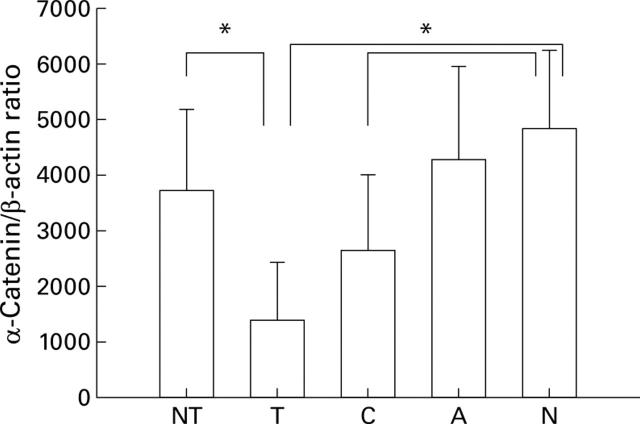
Figure 2 .
Semiquantitative RT-PCR analysis of α-catenin expression. NT, non-tumour; T, tumour; C, corpus from first degree relatives; A, antrum from first degree relatives; N, healthy subjects. *p<0.05.
Figure 3 .
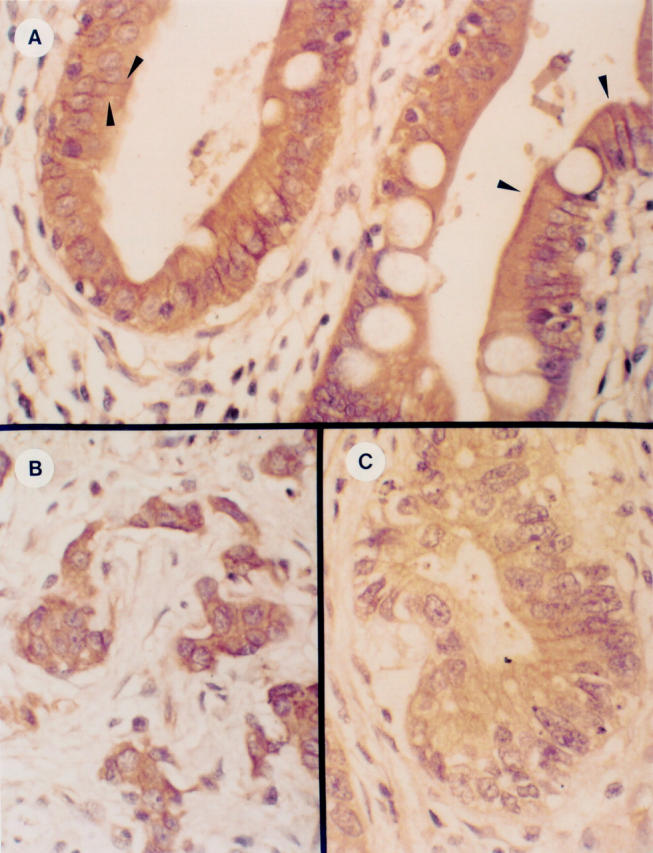
Immunohistochemical analysis of α-catenin expression. In the normal gastric epithelium, α-catenin immunoreactivity was located at the cell membrane (A, arrowheads) and in the cytoplasm. Gastric cancer cells exhibited various levels of cytoplasmic and/or membranous immunoreactivity in diffuse-type (B) or intestinal-type (C) gastric carcinoma. In some cases α-catenin immunoreactivity was decreased in the gastric cancer cells (C).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker K. F., Atkinson M. J., Reich U., Becker I., Nekarda H., Siewert J. R., Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994 Jul 15;54(14):3845–3852. [PubMed] [Google Scholar]
- Berx G., Becker K. F., Höfler H., van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12(4):226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Ebert M., Yokoyama M., Kobrin M. S., Friess H., Lopez M. E., Büchler M. W., Johnson G. R., Korc M. Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res. 1994 Aug 1;54(15):3959–3962. [PubMed] [Google Scholar]
- Fan X. G., Kelleher D., Fan X. J., Xia H. X., Keeling P. W. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut. 1996 Jan;38(1):19–22. doi: 10.1136/gut.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C. S., Mayer R. J. Gastric carcinoma. N Engl J Med. 1995 Jul 6;333(1):32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Nakatsuru S., Nagafuchi A., Tsukita S., Muto T., Nakamura Y., Horii A. Structure, expression and chromosome assignment of the human catenin (cadherin-associated protein) alpha 1 gene (CTNNA1). Cytogenet Cell Genet. 1994;65(1-2):74–78. doi: 10.1159/000133603. [DOI] [PubMed] [Google Scholar]
- Guilford P., Hopkins J., Harraway J., McLeod M., McLeod N., Harawira P., Taite H., Scoular R., Miller A., Reeve A. E. E-cadherin germline mutations in familial gastric cancer. Nature. 1998 Mar 26;392(6674):402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Jawhari A., Farthing M., Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue? Gut. 1997 Nov;41(5):581–584. doi: 10.1136/gut.41.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhari A., Jordan S., Poole S., Browne P., Pignatelli M., Farthing M. J. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997 Jan;112(1):46–54. doi: 10.1016/s0016-5085(97)70218-x. [DOI] [PubMed] [Google Scholar]
- Kekki M., Ihamäki T., Varis K., Siurala M. Chronic gastritis profiles in sibs of probands calculated to carry a highly increased risk of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;186:29–32. doi: 10.3109/00365529109103984. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Harney J. W., Larsen P. R. Studies of the hormonal regulation of type 2 5'-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998 Dec;139(12):4895–4905. doi: 10.1210/endo.139.12.6334. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992 Apr 17;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Negri E., Franceschi S., Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992 Jul 1;70(1):50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Matsui S., Shiozaki H., Inoue M., Tamura S., Doki Y., Kadowaki T., Iwazawa T., Shimaya K., Nagafuchi A., Tsukita S. Immunohistochemical evaluation of alpha-catenin expression in human gastric cancer. Virchows Arch. 1994;424(4):375–381. doi: 10.1007/BF00190559. [DOI] [PubMed] [Google Scholar]
- Matsuura K., Kawanishi J., Fujii S., Imamura M., Hirano S., Takeichi M., Niitsu Y. Altered expression of E-cadherin in gastric cancer tissues and carcinomatous fluid. Br J Cancer. 1992 Dec;66(6):1122–1130. doi: 10.1038/bjc.1992.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meining A., Hackelsberger A., Daenecke C., Stolte M., Bayerdörffer E., Ochsenkühn T. Increased cell proliferation of the gastric mucosa in first-degree relatives of gastric carcinoma patients. Cancer. 1998 Sep 1;83(5):876–881. [PubMed] [Google Scholar]
- Meining A., Stolte M., Hatz R., Lehn N., Miehlke S., Morgner A., Bayerdörffer E. Differing degree and distribution of gastritis in Helicobacter pylori-associated diseases. Virchows Arch. 1997 Jul;431(1):11–15. doi: 10.1007/s004280050063. [DOI] [PubMed] [Google Scholar]
- Miehlke S., Hackelsberger A., Meining A., von Arnim U., Müller P., Ochsenkühn T., Lehn N., Malfertheiner P., Stolte M., Bayerdörffer E. Histological diagnosis of Helicobacter pylori gastritis is predictive of a high risk of gastric carcinoma. Int J Cancer. 1997 Dec 10;73(6):837–839. doi: 10.1002/(sici)1097-0215(19971210)73:6<837::aid-ijc12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Nose A., Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992 Feb;116(4):989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierceall W. E., Woodard A. S., Morrow J. S., Rimm D., Fearon E. R. Frequent alterations in E-cadherin and alpha- and beta-catenin expression in human breast cancer cell lines. Oncogene. 1995 Oct 5;11(7):1319–1326. [PubMed] [Google Scholar]
- Pignatelli M., Vessey C. J. Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol. 1994 Sep;25(9):849–856. doi: 10.1016/0046-8177(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Sinard J. H., Morrow J. S. Reduced alpha-catenin and E-cadherin expression in breast cancer. Lab Invest. 1995 May;72(5):506–512. [PubMed] [Google Scholar]
- Rohwedel J., Guan K., Zuschratter W., Jin S., Ahnert-Hilger G., Fürst D., Fässler R., Wobus A. M. Loss of beta1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Dev Biol. 1998 Sep 15;201(2):167–184. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Nagafuchi A., Fujita S., Gotoh M., Takeichi M., Tsukita S., Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992 Oct 15;52(20):5770–5774. [PubMed] [Google Scholar]
- Tahara E., Semba S., Tahara H. Molecular biological observations in gastric cancer. Semin Oncol. 1996 Jun;23(3):307–315. [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Terrés A. M., Pajares J. M., O'Toole D., Ahern S., Kelleher D. H pylori infection is associated with downregulation of E-cadherin, a molecule involved in epithelial cell adhesion and proliferation control. J Clin Pathol. 1998 May;51(5):410–412. doi: 10.1136/jcp.51.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T. The cadherin superfamily at the synapse: more members, more missions. Cell. 1998 Jun 26;93(7):1095–1098. doi: 10.1016/s0092-8674(00)81452-x. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Some structural and functional aspects of the cell adhesion molecule uvomorulin. Cell Differ. 1984 Dec;15(2-4):269–273. doi: 10.1016/0045-6039(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Zanghieri G., Di Gregorio C., Sacchetti C., Fante R., Sassatelli R., Cannizzo G., Carriero A., Ponz de Leon M. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer. 1990 Nov 1;66(9):2047–2051. doi: 10.1002/1097-0142(19901101)66:9<2047::aid-cncr2820660934>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]