Abstract
BACKGROUND—The development of macrolide resistance in Helicobacter pylori is considered an essential reason for failure of antibiotic eradication therapies. The predominant mechanism of resistance to macrolides, particularly clarithromycin, is based on three defined mutations within 23S rRNA, resulting in decreased binding of the antibiotic to the bacterial ribosome. AIM—To develop an rRNA based whole cell hybridisation method to detect Helicobacter species in situ within gastric tissue, simultaneously with its clarithromycin resistance genotype. METHODS—A set of fluorescent labelled oligonucleotide probes was developed, binding either to H pylori 16S rRNA or 23S rRNA sequences containing specific point mutations responsible for clarithromycin resistance. After hybridisation and stringent washing procedures, labelling of intact single bacteria was monitored by fluorescence microscopy. The new approach was compared with PCR based assays, histology, and microbiological culture. RESULTS—In comparison with the phenotypic resistance measurement by E test, the genotypic clarithromycin resistance correlated perfectly (100%) for 35 H pylori isolates analysed. In a set of gastric biopsy specimens (27) H pylori infection was confirmed by histology (17/27) and correctly detected by whole cell hybridisation. Five clarithromycin resistant strains were identified in gastric tissue specimens directly. Furthermore, non-cultivable coccoid forms of H pylori were easily detectable by whole cell hybridisation. CONCLUSIONS—Whole cell hybridisation of rRNA holds great promise for cultivation independent, reliable, and rapid (three hours) genotypic determination of clarithromycin resistance in H pylori. Compared with PCR techniques it is independent of nucleic acid preparations, not prone to inhibition, and allows semiquantitative visualisation of the bacteria within intact tissue samples. Keywords: Helicobacter pylori; macrolide resistance; clarithromycin; in situ hybridisation; genotypic resistance
Full Text
The Full Text of this article is available as a PDF (210.4 KB).
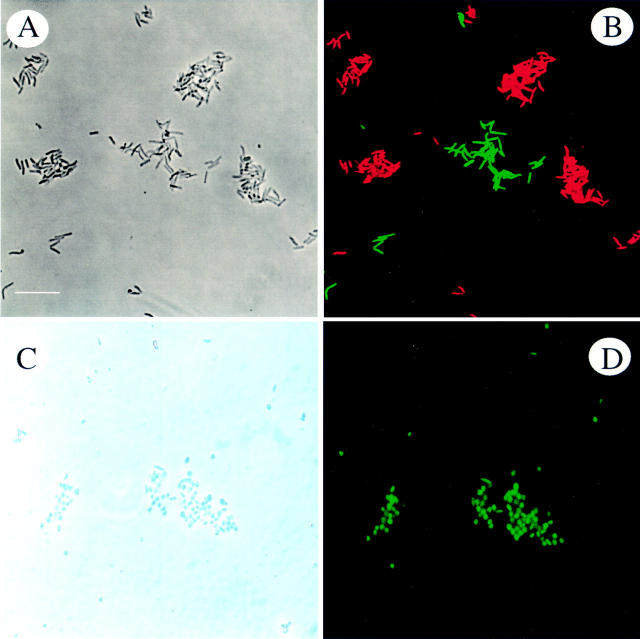
Figure 1 .
Specificity testing of oligonucleotide probe Hpy-1 for discrimination of H pylori from related helicobacters. (A) A mixture of H pylori and H mustelae, as seen by phase contrast microscopy. (B) Detection of the bacterial mixture with two probes, a H pylori specific probe Hpy-1-Cy3 (red) and a bacterial specific probe Eub-FLUOS (green). Both helicobacters bound the bacterial probe (green) but H pylori bound Hpy-1-Cy3 (red) in addition, resulting in the yellow appearance of H pylori (mixed colour). Bar represents 10 µm.
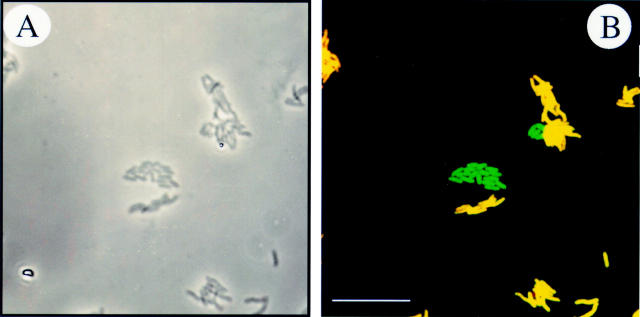
Figure 2 .
Specific detection of clarithromycin sensitive and resistant H pylori isolates. (A) Phase contrast microscopy of a mixture of clarithromycin sensitive and resistant H pylori isolates. (B) The same microscopic field after hybridisation with probes ClaWT-FLUOS (green) and ClaR1-Cy3 (red). (C) Phase contrast micrograph showing coccoid forms of clarithromycin resistant H pylori. (D) Whole cell hybridisation of coccoid forms with probe Hpy-1-FLUOS. Bar represents 10 µm.
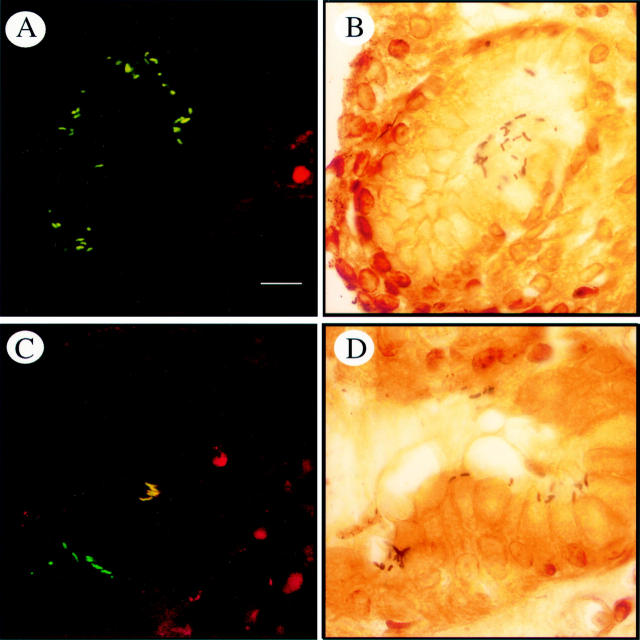
Figure 3 .
Specific detection of clarithromycin sensitive and resistant H pylori isolates within gastric antrum sections of H pylori infected patients. (Left) Detection of H pylori and clarithromycin resistance by whole cell hybridisation with fluorescent probes Hpy-1-FLUOS and ClaR1-Cy3 simultaneously. (Right) Antrum sections from the same biopsy material (Warthin-Starry stain). (A) Clarithromycin resistant H pylori are visible in yellow (mixed colour between green and red). (C) Mixed population of clarithromycin resistant (yellow) and sensitive (green) H pylori in the same biopsy specimen of a patient. (B, D) Antrum sections showing H pylori by the Warthin-Starry stain, which does not allow resistance determination.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997 Sep 1;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. I., Krumholz L., Stahl D. A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990 Feb;172(2):762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axon A. T., Moayyedi P. Eradication of Helicobacter pylori: omeprazole in combination with antibiotics. Scand J Gastroenterol Suppl. 1996;215:82–89. [PubMed] [Google Scholar]
- Blaser M. J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992 Feb;102(2):720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Cirillo D., Kagnoff M. F., Guiney D. G., Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997 Feb;65(2):843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets-Ossenkopp Y. J., Sparrius M., Kusters J. G., Kolkman J. J., Vandenbroucke-Grauls C. M. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996 Aug 15;142(1):37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Dunn B. E., Cohen H., Blaser M. J. Helicobacter pylori. Clin Microbiol Rev. 1997 Oct;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arata M. I., Baquero F., de Rafael L., Martín de Argila C., Gisbert J. P., Bermejo F., Boixeda D., Cantón R. Mutations in 23S rRNA in Helicobacter pylori conferring resistance to erythromycin do not always confer resistance to clarithromycin. Antimicrob Agents Chemother. 1999 Feb;43(2):374–376. doi: 10.1128/aac.43.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998 Nov;115(5):1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- Hultén K., Gibreel A., Sköld O., Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997 Nov;41(11):2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen T. J., Genta R. M., Yoffe B., Hachem C. Y., Graham D. Y., el-Zaatari F. A. Detection of Helicobacter pylori in paraffin-embedded gastric biopsy specimens by in situ hybridization. Am J Clin Pathol. 1996 Sep;106(3):305–311. doi: 10.1093/ajcp/106.3.305. [DOI] [PubMed] [Google Scholar]
- Lind T., Veldhuyzen van Zanten S., Unge P., Spiller R., Bayerdörffer E., O'Morain C., Bardhan K. D., Bradette M., Chiba N., Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996 Sep;1(3):138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Marais A., Monteiro L., Occhialini A., Pina M., Lamouliatte H., Mégraud F. Direct detection of Helicobacter pylori resistance to macrolides by a polymerase chain reaction/DNA enzyme immunoassay in gastric biopsy specimens. Gut. 1999 Apr;44(4):463–467. doi: 10.1136/gut.44.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F. Antibiotic resistance in Helicobacter pylori infection. Br Med Bull. 1998;54(1):207–216. doi: 10.1093/oxfordjournals.bmb.a011671. [DOI] [PubMed] [Google Scholar]
- Mégraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998 Nov;115(5):1278–1282. doi: 10.1016/s0016-5085(98)70101-5. [DOI] [PubMed] [Google Scholar]
- Occhialini A., Urdaci M., Doucet-Populaire F., Bébéar C. M., Lamouliatte H., Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997 Dec;41(12):2724–2728. doi: 10.1128/aac.41.12.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina M., Occhialini A., Monteiro L., Doermann H. P., Mégraud F. Detection of point mutations associated with resistance of Helicobacter pylori to clarithromycin by hybridization in liquid phase. J Clin Microbiol. 1998 Nov;36(11):3285–3290. doi: 10.1128/jcm.36.11.3285-3290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G. G., Shortridge D., Versalovic J., Beyer J., Flamm R. K., Graham D. Y., Ghoneim A. T., Tanaka S. K. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997 Mar;41(3):712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczebara F., Dhaenens L., Vincent P., Husson M. O. Evaluation of rapid molecular methods for detection of clarithromycin resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1997 Feb;16(2):162–164. doi: 10.1007/BF01709478. [DOI] [PubMed] [Google Scholar]
- Trebesius K., Harmsen D., Rakin A., Schmelz J., Heesemann J. Development of rRNA-targeted PCR and in situ hybridization with fluorescently labelled oligonucleotides for detection of Yersinia species. J Clin Microbiol. 1998 Sep;36(9):2557–2564. doi: 10.1128/jcm.36.9.2557-2564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Shortridge D., Kibler K., Griffy M. V., Beyer J., Flamm R. K., Tanaka S. K., Graham D. Y., Go M. F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996 Feb;40(2):477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon A. C., Doglioni C., Diss T. C., Pan L., Moschini A., de Boni M., Isaacson P. G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993 Sep 4;342(8871):575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- van Zwet A. A., Vandenbroucke-Grauls C. M., Thijs J. C., van der Wouden E. J., Gerrits M. M., Kusters J. G., Vandenbrouke-Grauls C. M. Stable amoxicillin resistance in Helicobacter pylori. Lancet. 1998 Nov 14;352(9140):1595–1595. doi: 10.1016/s0140-6736(98)00064-6. [DOI] [PubMed] [Google Scholar]