Abstract
BACKGROUND AND AIMS—Gastric metaplasia is frequently seen in biopsies of the duodenal cap, particularly when inflamed or ulcerated. In its initial manifestation small patches of gastric foveolar cells appear near the tip of a villus. These cells contain periodic acid-Schiff (PAS) positive neutral mucins in contrast with the alcian blue (AB) positive acidic mucins within duodenal goblet cells. Previous investigations have suggested that these PAS positive cells originate either in Brunner's gland ducts or at the base of duodenal crypts and migrate in distinct streams to the upper villus. To investigate the origin of gastric metaplasia in superficial patches, we used the PAS/AB stain to distinguish between neutral and acidic mucins and in addition specific antibodies to immunolocalise foveolar cell mucin MUC5AC, the foveolar cell secretory product, gastric trefoil factor (TFF1), the mature goblet cell mucin MUC2, and MUC2 core antigen. RESULTS—Cells in focal patches of gastric metaplasia contained secretory granules of both gastric and goblet cell phenotypes. MUC5AC and TFF1 were present as expected in gastric foveolar cells but in addition, MUC2 core antigen, normally present only in the Golgi of intestinal goblet cells, was expressed in secretory granules. Goblet cells in the vicinity of metaplastic patches also expressed both gastric and intestinal antigens. MUC5AC/MUC2 containing goblet cells were most common near the villus tip but were also seen at the base of crypts. Where crypts and Brunner's gland ducts merged they were always seen on the crypt side of the junction. Goblet cells were the only cells to express gastric antigens in these areas. In advanced metaplastic lesions, dual phenotype goblet cells were less evident and fewer cells expressed intestinal mucin antigens. CONCLUSIONS—We suggest that goblet cells that express both intestinal and gastric antigens may represent local precursors of gastric metaplasia undergoing a transition to foveolar-like cells of mixed phenotype at the site of early metaplastic patches. As metaplasia becomes more widespread, a more pure gastric phenotype emerges. This progression is likely to be controlled by local inflammatory signals. Keywords: gastric metaplasia; goblet cells; mucin
Full Text
The Full Text of this article is available as a PDF (340.4 KB).
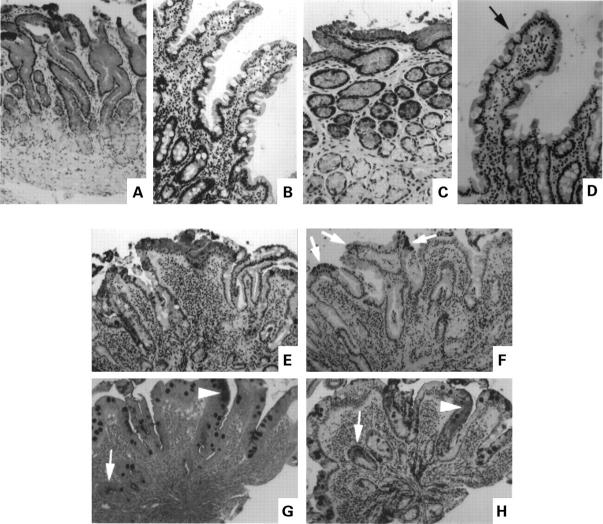
Figure 1 .
Immunohistochemical localisation of the gastric mucin MUC5AC and gastric trefoil factor (TFF1) in normal antrum and duodenum (A-D), and duodenum containing focal gastric metaplasia (E, F, and H). (A) MUC5AC, normal antrum; (B) MUC5AC, normal duodenum; (C) TFF1 in the same area of the antral biopsy shown in (A);(D) TFF1, normal duodenum, arrow marks a weakly stained goblet cell; (E, F) serial sections of duodenum containing gastric metaplasia ((E) MUC5AC, (F) TFF1), arrows show positively stained areas; (G, H) serial sections stained by PAS/AB (G) and for MUC5AC (H), arrows point to sites of PAS staining; in (G), all goblet cells were AB positive.
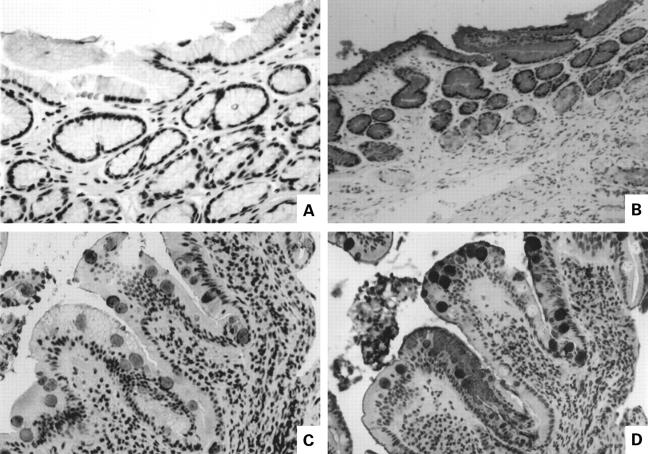
Figure 2 .
Distribution of intestinal mucin MUC2 and gastric mucin MUC5AC in serial sections of antrum and duodenum. (A) MUC2, normal antrum; (B) MUC5AC, normal antrum; (C, D) duodenum containing focal gastric metaplasia ((C) MUC2; (D) MUC5AC).
Figure 3 .
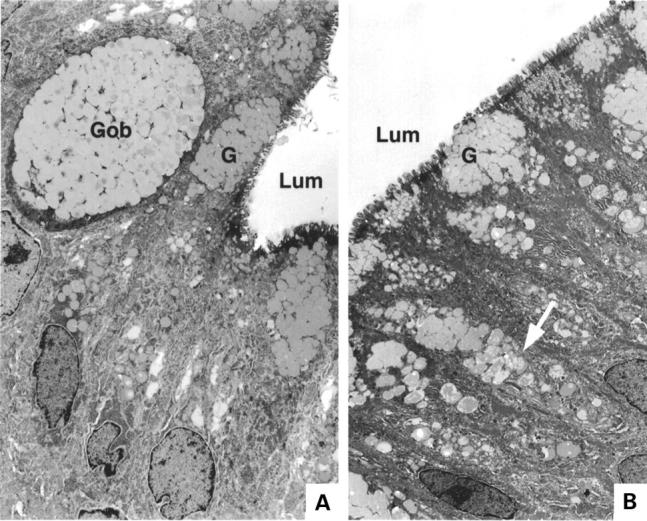
Electron micrograph of two areas within a patch of focal metaplasia. In each, Lum=lumenal surface. (A) Gob, a cell containing mucous secretory granules typical of intestinal goblet cells; G, mucus secretory granules typical of gastric foveolar cells. (B) Arrow shows single cell containing granules typical of both gastric and intestinal phenotypes; G, mucus granules with gastric phenotype.
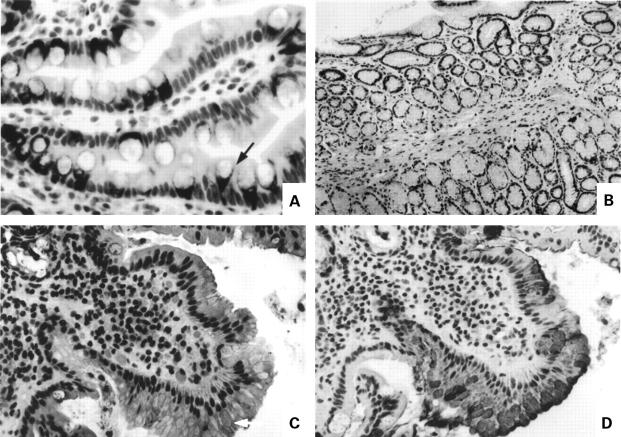
Figure 4 .
Immunolocalisation of MUC2 core antigen. (A) Normal duodenum showing staining for core antigen in the supranuclear Golgi region at the base of the goblet cell storage granule mass (arrow); (B) absence of core antigen staining in normal antrum; (C) MUC2 core antigen in the secretory granules of cells in a focal area of duodenal gastric metaplasia (arrow); (D) MUC5AC staining of area shown in (C).
Figure 5 .
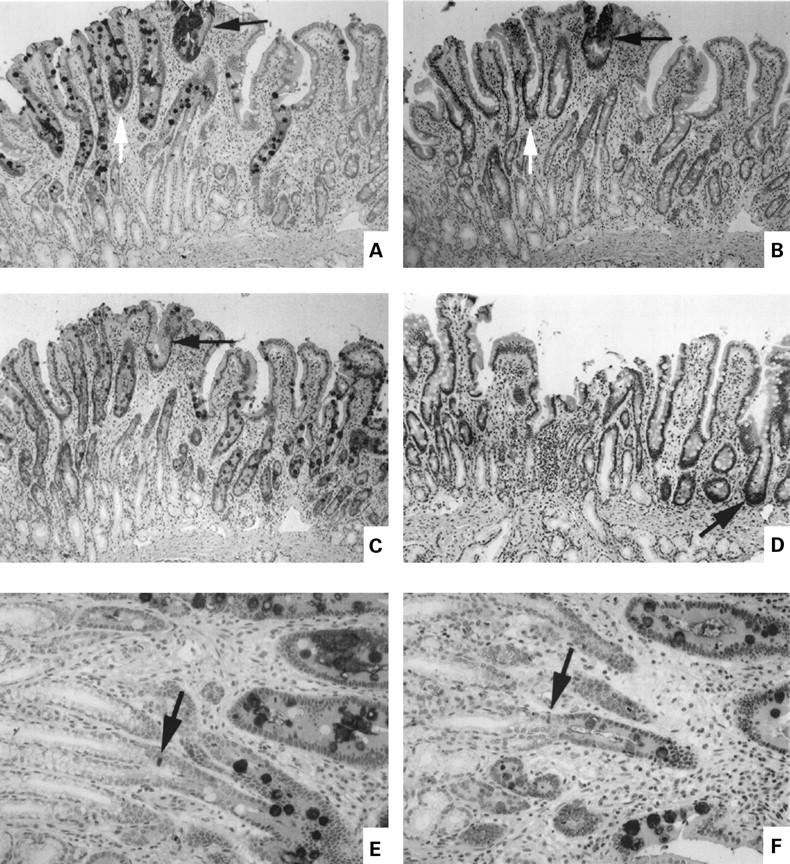
Expression of gastric and intestinal markers in cells near a patch of focal duodenal gastric metaplasia. Panels A-D are serial sections of well oriented mucosa showing entire crypt-villus unit plus junctional zone between crypts and Brunner's gland ducts. (A) MUC5AC; (B) gastric trefoil factor (TFF1); (C) MUC2; (D) human defensin 5 (HD5). The arrows in (A), (B), and (C) show an area of metaplasia. In (D), the arrow shows a positively stained Paneth's cell at the base of a crypt. (E, F) Serial sections at higher magnification of the junctional zone between Brunner's gland ducts and crypts ((E) MUC5AC; (F) MUC 2). The arrows show a stained cell of indeterminate phenotype at the junction of crypt and duct.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Poulsom R., Stamp G. W., Elia G., Pike C., Jeffery R., Longcroft J., Rio M. C., Chambon P., Wright N. A. The ulceration-associated cell lineage (UACL) reiterates the Brunner's gland differentiation programme but acquires the proliferative organization of the gastric gland. J Pathol. 1994 Aug;173(4):317–326. doi: 10.1002/path.1711730406. [DOI] [PubMed] [Google Scholar]
- Cheli R., Cornaggia M., Testino G., De Iaco F. Gastric metaplasia in normal (inflammation-free) duodenal mucosa. J Clin Gastroenterol. 1994 Apr;18(3):240–241. doi: 10.1097/00004836-199404000-00016. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Dooley C. P., Cohen H., Appleman M. D. Prevalence of gastric metaplasia, inflammation, and Campylobacter pylori in the duodenum of members of a normal population. Am J Clin Pathol. 1988 Dec;90(6):711–714. doi: 10.1093/ajcp/90.6.711. [DOI] [PubMed] [Google Scholar]
- Frierson H. F., Jr, Caldwell S. H., Marshall B. J. Duodenal bulb biopsy findings for patients with non-ulcer dyspepsia with or without Campylobacter pylori gastritis. Mod Pathol. 1990 May;3(3):271–276. [PubMed] [Google Scholar]
- Gormally S. M., Kierce B. M., Daly L. E., Bourke B., Carroll R., Durnin M. T., Drumm B. Gastric metaplasia and duodenal ulcer disease in children infected by Helicobacter pylori. Gut. 1996 Apr;38(4):513–517. doi: 10.1136/gut.38.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Elia G., Singh S., Longcroft J. M., Wright N. A. The expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in 'gastric metaplasia' of the proximal duodenum: implications for the nature of 'gastric metaplasia'. J Pathol. 1993 Mar;169(3):355–360. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Gummett P. A., Walker M. M., Misiewicz J. J., Baron J. H. Relation between gastric acid output, Helicobacter pylori, and gastric metaplasia in the duodenal bulb. Gut. 1996 Oct;39(4):513–520. doi: 10.1136/gut.39.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. H. GASTRIC EPITHELIUM IN THE DUODENUM. Gut. 1964 Aug;5:285–294. doi: 10.1136/gut.5.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K., Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J. 1997 Jul;10(7):1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- Lahoti C., Thorner P., Malkin D., Yeger H. Immunohistochemical detection of p53 in Wilms' tumors correlates with unfavorable outcome. Am J Pathol. 1996 May;148(5):1577–1589. [PMC free article] [PubMed] [Google Scholar]
- Lechago J., Black C., Samloff I. M. Immunofluorescence studies of gastric heterotopia of the small intestine in Crohn's disease. Gastroenterology. 1976 Mar;70(3):429–432. [PubMed] [Google Scholar]
- Liu K. C., Wright N. A. The migration pathway of epithelial cells on human duodenal villi: the origin and fate of 'gastric metaplastic' cells in duodenal mucosa. Epithelial Cell Biol. 1992 Apr;1(2):53–58. [PubMed] [Google Scholar]
- McCool D. J., Forstner J. F., Forstner G. G. Synthesis and secretion of mucin by the human colonic tumour cell line LS180. Biochem J. 1994 Aug 15;302(Pt 1):111–118. doi: 10.1042/bj3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S., Calam J. Helicobacter pylori and peptic ulcers: the present position. Gut. 1992 Mar;33(3):289–292. doi: 10.1136/gut.33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick W. J., Denham D., Forrest A. P. Mucous change in the human duodenum: a light and electron microscopic study and correlation with disease and gastric acid secretion. Gut. 1974 Oct;15(10):767–776. doi: 10.1136/gut.15.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Poulsom R., Chinery R., Sarraf C., Lalani E. N., Stamp G., Elia G., Wright N. Trefoil peptide expression in intestinal adaptation and renewal. Scand J Gastroenterol Suppl. 1992;192:17–28. doi: 10.3109/00365529209095975. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Shabib S. M., Cutz E., Drumm B., Sherman P. M. Association of gastric metaplasia and duodenitis with Helicobacter pylori infection in children. Am J Clin Pathol. 1994 Aug;102(2):188–191. doi: 10.1093/ajcp/102.2.188. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Shousha S., Parkins R. A., Bull T. B. Chronic duodenitis with gastric metaplasia: electron microscopic study including comparison with normal. Histopathology. 1983 Nov;7(6):873–885. doi: 10.1111/j.1365-2559.1983.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Sotozono M., Okada Y., Sasagawa T., Nakatou T., Yoshida A., Yokoi T., Kubota M., Tsuji T. Novel monoclonal antibody, SO-MU1, against human gastric MUC5AC apomucin. J Immunol Methods. 1996 Jun 10;192(1-2):87–96. doi: 10.1016/0022-1759(96)00025-7. [DOI] [PubMed] [Google Scholar]
- Wright N. A. Migration of the ductular elements of gut-associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: the origins of "gastric metaplasia" in the duodenum of the specialized mucosa of barrett's esophagus and of pseudopyloric metaplasia. Yale J Biol Med. 1996 Mar-Apr;69(2):147–153. [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Pike C. M., Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion. 1990;46 (Suppl 2):125–133. doi: 10.1159/000200375. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Sobala G. M., Shallcross T., Heatley R. V., Axon A. T., Dixon M. F. Gastric epithelium in the duodenum: its association with Helicobacter pylori and inflammation. J Clin Pathol. 1990 Dec;43(12):981–986. doi: 10.1136/jcp.43.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama I., Kozuka S., Ito K., Kubota K., Yokoyama Y. Gastric gland metaplasia in the small and large intestine. Gut. 1977 Mar;18(3):214–218. doi: 10.1136/gut.18.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]