Abstract
BACKGROUND—The bacterium Helicobacter pylori is able to adhere to and to colonise the human gastric epithelium, yet the primary gene product responsible as a receptor for its adherence has not been identified. AIMS—To investigate the expression of the gastric mucins MUC5AC and MUC6 in the gastric epithelium in relation to H pylori colonisation in order to examine their possible roles in the binding of H pylori. PATIENTS—Seventy two consecutive patients suspected of having H pylori infection. METHODS—MUC5AC, MUC6, and H pylori were detected in single sections of antral biopsy specimens using immunohistochemical triple staining. RESULTS—MUC5AC was expressed in the superficial epithelium and the upper part of the gastric pits. MUC6 expression was detected in the lower part of the gastric pits. The expression of both mucins in the epithelium was complementary. In each patient, there was a sharply delineated transition between MUC5AC and MUC6 producing cell populations. In all H pylori positive patients there was a striking colocalisation of H pylori and MUC5AC; more than 99% of the bacteria were associated with either extracellular MUC5AC or the apical domain of MUC5AC producing cells. CONCLUSIONS—H pylori is very closely associated with extracellular MUC5AC and epithelial cells that produce MUC5AC. This indicates that MUC5AC, but not MUC6, plays a role in the adhesion of H pylori to the gastric mucosa. Keywords: Helicobacter pylori; gastric mucin; MUC5AC; MUC6; stomach
Full Text
The Full Text of this article is available as a PDF (199.7 KB).
Figure 1 .
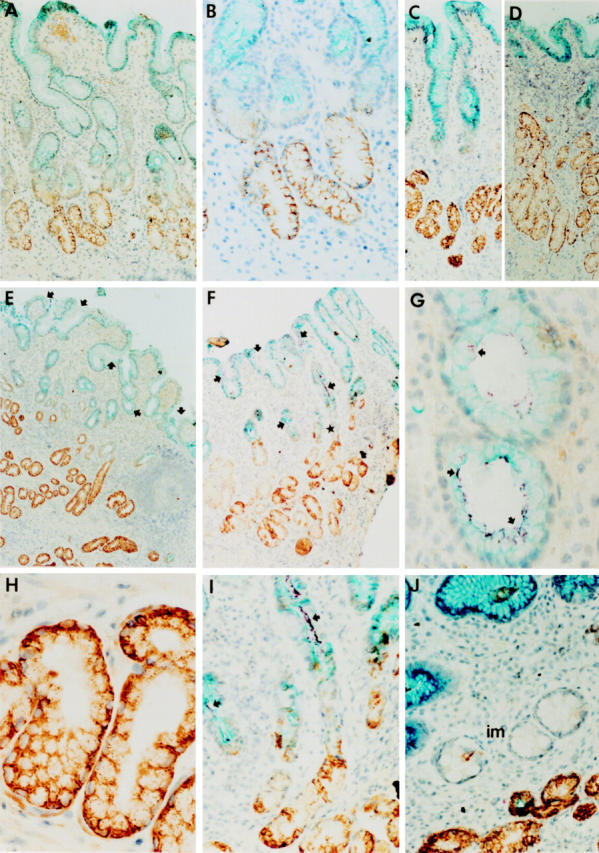
Immunohistochemical staining of sections of human stomach. All sections were immunohistochemically triple stained using antibodies against MUC5AC, MUC6, and H pylori. Detection of MUC5AC yielded a turquoise blue colour. MUC6 was detected using a DAB staining, staining MUC6 brown. Detection of H pylori yielded a reddish purple staining. All sections were counterstained with haematoxylin. (A) Section from an individual without H pylori infection. Note the complementary expression of MUC5AC and MUC6 within the epithelium. There is some yellow/orange staining in the mucosa. This is caused by erythrocytes that appear yellow/orange in this particular quadruple staining. (B) Higher magnification of A showing the transition zone of MUC5AC and MUC6 expression. (C) Distribution of MUC5AC and MUC6 along the pit-gland axis in a H pylori negative patient. (D) A H pylori positive patient at the same magnification as C (the bacteria cannot be discerned at this magnification in this patient). Notice in C and D that the glands have approximately the same length, but that the number of MUC6 expressing cells has increased, whereas the number of MUC5AC expressing cells has diminished. (E) and (F) Sections of H pylori positive patients, showing heavy infection of the mucus layer and epithelium. The star indicates a gland that is shown at higher magnification in panel I. (G-I) Higher magnifications of sections from H pylori positive patients. Notice in E-I that the H pylori bacteria colocalise with MUC5AC and with MUC5AC producing cells, but not with MUC6. (J) Section of a H pylori negative patient (after successful eradication of the bacterium), showing a focal area of intestinal metaplasia (im). Notice that the areas of intestinal metaplasia contain characteristic "intestinal type" goblet cells, but stained neither for MUC5AC nor MUC6. Arrows in E-G and I indicate purple stained, clustered H pylori bacteria. In all patients the detection of H pylori in the sections was in accordance with the culturing of the bacterium and routine histology using H&E staining. Original magnification × 62.5 (A); × 125 (B); × 62.5 (C); × 62.5 (D); × 31 (E); × 31 (F); × 250 (G); × 250 (H); × 50 (I); × 125 (J).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Simoons-Smit I., Negrini R., Moran A. P., Aspinall G. O., Forte J. G., De Vries T., Quan H., Verboom T., Maaskant J. J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996 Jun;64(6):2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara J., Chastre E., Mahiou J., Singh R. L., Forgue-Lafitte M. E., Hollande E., Godeau F. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer. 1998 Mar 2;75(5):767–773. doi: 10.1002/(sici)1097-0215(19980302)75:5<767::aid-ijc17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bobek L. A., Tsai H., Biesbrock A. R., Levine M. J. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem. 1993 Sep 25;268(27):20563–20569. [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Yan P., Sternberg L., Yunker C. K., Scheiman J. M., Bresalier R. S. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997 Aug;113(2):455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- Carrick J., Lee A., Hazell S., Ralston M., Daskalopoulos G. Campylobacter pylori, duodenal ulcer, and gastric metaplasia: possible role of functional heterotopic tissue in ulcerogenesis. Gut. 1989 Jun;30(6):790–797. doi: 10.1136/gut.30.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne M., Drumm B. Absence of effect of Lewis A and Lewis B expression on adherence of Helicobacter pylori to human gastric cells. Gastroenterology. 1997 Jul;113(1):72–80. doi: 10.1016/s0016-5085(97)70082-9. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Triadafilopoulos G. Blood group-related antigen expression in normal and metaplastic human upper gastrointestinal mucosa. Gastroenterology. 1992 Nov;103(5):1552–1561. doi: 10.1016/0016-5085(92)91177-6. [DOI] [PubMed] [Google Scholar]
- De Bolós C., Garrido M., Real F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995 Sep;109(3):723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- Dekker J., Aelmans P. H., Strous G. J. The oligomeric structure of rat and human gastric mucins. Biochem J. 1991 Jul 15;277(Pt 2):423–427. doi: 10.1042/bj2770423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Drumm B., Sherman P., Cutz E., Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987 Jun 18;316(25):1557–1561. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- Dye K. R., Marshall B. J., Frierson H. F., Jr, Pambianco D. J., McCallum R. W. Campylobacter pylori colonizing heterotopic gastric tissue in the rectum. Am J Clin Pathol. 1990 Jan;93(1):144–147. doi: 10.1093/ajcp/93.1.144. [DOI] [PubMed] [Google Scholar]
- Guruge J. L., Falk P. G., Lorenz R. G., Dans M., Wirth H. P., Blaser M. J., Berg D. E., Gordon J. I. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Ho S. B., Niehans G. A., Lyftogt C., Yan P. S., Cherwitz D. L., Gum E. T., Dahiya R., Kim Y. S. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993 Feb 1;53(3):641–651. [PubMed] [Google Scholar]
- Ho S. B., Roberton A. M., Shekels L. L., Lyftogt C. T., Niehans G. A., Toribara N. W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995 Sep;109(3):735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Ho S. B., Shekels L. L., Toribara N. W., Kim Y. S., Lyftogt C., Cherwitz D. L., Niehans G. A. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995 Jun 15;55(12):2681–2690. [PubMed] [Google Scholar]
- Ilver D., Arnqvist A., Ogren J., Frick I. M., Kersulyte D., Incecik E. T., Berg D. E., Covacci A., Engstrand L., Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998 Jan 16;279(5349):373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- Kenny B., DeVinney R., Stein M., Reinscheid D. J., Frey E. A., Finlay B. B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997 Nov 14;91(4):511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Sakamoto J., Kito T., Yamamura Y., Koshikawa T., Fujita M., Watanabe T., Nakazato H. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma, and gastric carcinoma. Am J Gastroenterol. 1993 Jun;88(6):919–924. [PubMed] [Google Scholar]
- Mack D. R., Blain-Nelson P. L. Disparate in vitro inhibition of adhesion of enteropathogenic Escherichia coli RDEC-1 by mucins isolated from various regions of the intestinal tract. Pediatr Res. 1995 Jan;37(1):75–80. doi: 10.1203/00006450-199501000-00015. [DOI] [PubMed] [Google Scholar]
- Mollicone R., Bara J., Le Pendu J., Oriol R. Immunohistologic pattern of type 1 (Lea, Leb) and type 2 (X, Y, H) blood group-related antigens in the human pyloric and duodenal mucosae. Lab Invest. 1985 Aug;53(2):219–227. [PubMed] [Google Scholar]
- Moran A. P. Cell surface characteristics of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995 Feb;10(3-4):271–280. doi: 10.1111/j.1574-695X.1995.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Morris A., Nicholson G., Zwi J., Vanderwee M. Campylobacter pylori infection in Meckel's diverticula containing gastric mucosa. Gut. 1989 Sep;30(9):1233–1235. doi: 10.1136/gut.30.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Egami H., Shibata Y., Sakamoto K., Misumi A., Ogawa M. Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am J Clin Pathol. 1992 Jul;98(1):67–75. doi: 10.1093/ajcp/98.1.67. [DOI] [PubMed] [Google Scholar]
- Mégraud F., Brassens-Rabbé M. P., Denis F., Belbouri A., Hoa D. Q. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989 Aug;27(8):1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzenmaier A., Lange C., Glocker E., Covacci A., Moran A., Bereswill S., Baeuerle P. A., Kist M., Pahl H. L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J Immunol. 1997 Dec 15;159(12):6140–6147. [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Oberhuber G., Kranz A., Dejaco C., Dragosics B., Mosberger I., Mayr W., Radaszkiewicz T. Blood groups Lewis(b) and ABH expression in gastric mucosa: lack of inter-relation with Helicobacter pylori colonisation and occurrence of gastric MALT lymphoma. Gut. 1997 Jul;41(1):37–42. doi: 10.1136/gut.41.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Peterson W. L. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991 Apr 11;324(15):1043–1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Development of anti-human colonic mucin monoclonal antibodies. Characterization of multiple colonic mucin species. J Clin Invest. 1986 Apr;77(4):1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C. A., David L., Nielsen P. A., Clausen H., Mirgorodskaya K., Roepstorff P., Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997 Feb 20;74(1):112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Segal E. D., Falkow S., Tompkins L. S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. D., Lange C., Covacci A., Tompkins L. S., Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. A., Tummuru M. K., Blaser M. J., Kerr L. D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998 Mar 1;160(5):2401–2407. [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Pilus-mediated interactions of the Escherichia coli strain RDEC-1 with mucosal glycoproteins in the small intestine of rabbits. Gastroenterology. 1987 Oct;93(4):734–743. doi: 10.1016/0016-5085(87)90435-5. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Kaper J. B., Mack D. R. Intestinal mucin inhibits adhesion of human enteropathogenic Escherichia coli to HEp-2 cells. J Pediatr Gastroenterol Nutr. 1995 Oct;21(3):269–276. doi: 10.1097/00005176-199510000-00004. [DOI] [PubMed] [Google Scholar]
- Steer H. W. Surface morphology of the gastroduodenal mucosa in duodenal ulceration. Gut. 1984 Nov;25(11):1203–1210. doi: 10.1136/gut.25.11.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Tytgat K. M., Büller H. A., Opdam F. J., Kim Y. S., Einerhand A. W., Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994 Nov;107(5):1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis L. S., Mentis A. F., Makris A. M., Spiliadis C., Blackwell C., Weir D. M. In vitro binding of Helicobacter pylori to human gastric mucin. Infect Immun. 1991 Nov;59(11):4252–4254. doi: 10.1128/iai.59.11.4252-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Klinken B. J., Dekker J., Büller H. A., Einerhand A. W. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995 Nov;269(5 Pt 1):G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Dixon M. F., Heatley R. V. Campylobacter pyloridis and acid induced gastric metaplasia in the pathogenesis of duodenitis. J Clin Pathol. 1987 Aug;40(8):841–848. doi: 10.1136/jcp.40.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca E., Bertoni F., Roggero E., Bosshard G., Cazzaniga G., Pedrinis E., Biondi A., Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998 Mar 19;338(12):804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]
- de Cothi G. A., Newbold K. M., O'Connor H. J. Campylobacter-like organisms and heterotopic gastric mucosa in Meckel's diverticula. J Clin Pathol. 1989 Feb;42(2):132–134. doi: 10.1136/jcp.42.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klinken B. J., Dekker J., van Gool S. A., van Marle J., Büller H. A., Einerhand A. W. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998 May;274(5 Pt 1):G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]


