Abstract
BACKGROUND—Glucagon-like peptide-1(7-36)amide (GLP-1) is a gut hormone released postprandially. Synthetic GLP-1 strongly inhibits gastric emptying in healthy subjects and in patients with diabetes mellitus. AIMS—To investigate the effects of GLP-1 on antro-pyloro-duodenal motility in humans. METHODS—Eleven healthy male volunteers were studied on two separate days. On the interdigestive study day, a basal period was followed by a 60 minute period of saline infusion and two further 60 minute periods of intravenous infusion of GLP-1 0.4 and 1.2 pmol/kg/min to achieve postprandial and supraphysiological plasma levels, respectively. On the postprandial study day, the same infusions were coadministered with intraduodenal lipid perfusion at 2.5 ml/min (2.5 kcal/min) followed by another 60 minutes of recording after cessation of GLP-1. Antro-pyloro-duodenal motility was measured by perfusion manometry. RESULTS—GLP-1 significantly inhibited the number and amplitudes of antral and duodenal contractions in the interdigestive state and after administration of duodenal lipid. It abolished interdigestive antral wave propagation. In the interdigestive state, GLP-1 dose dependently increased pyloric tone and significantly stimulated isolated pyloric pressure waves (IPPW). Pyloric tone increased with duodenal lipid, and this was further enhanced by GLP-1. GLP-1 transiently restored the initial IPPW response to duodenal lipid which had declined with lipid perfusion. Plasma levels of pancreatic polypeptide were dose dependently diminished by GLP-1 with and without duodenal lipid. CONCLUSIONS—GLP-1 inhibited antro-duodenal contractility and stimulated the tonic and phasic motility of the pylorus. These effects probably mediate delayed gastric emptying. Inhibition of efferent vagal activity may be an important mechanism. As postprandial plasma levels of GLP-1 are sufficient to appreciably affect motility, we believe that endogenous GLP-1 is a physiological regulator of motor activity in the antro-pyloro-duodenal region. Keywords: GLP-1; gastrointestinal motility; pylorus; pancreatic polypeptide
Full Text
The Full Text of this article is available as a PDF (241.4 KB).
Figure 1 .
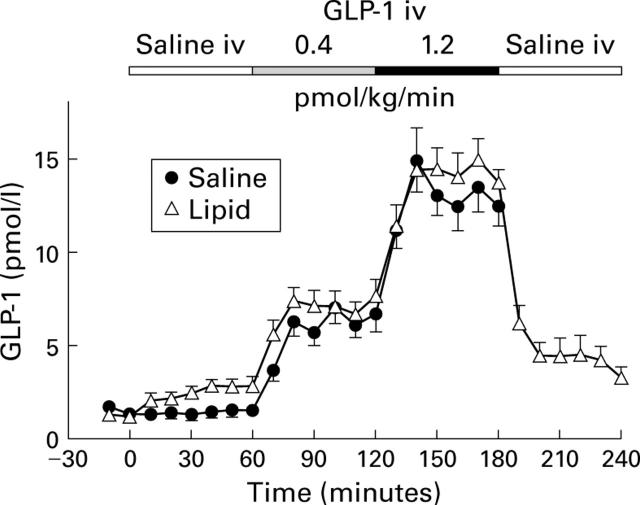
Plasma immunoreactivities of GLP-1 in response to intravenous infusions of saline, and GLP-1(7-36)amide 0.4 and 1.2 pmol/kg/min with concomitant duodenal perfusion of saline or lipid 2.5 kcal/min in 11 healthy volunteers (mean (SEM)). For statistical analysis, see table 1.
Figure 2 .
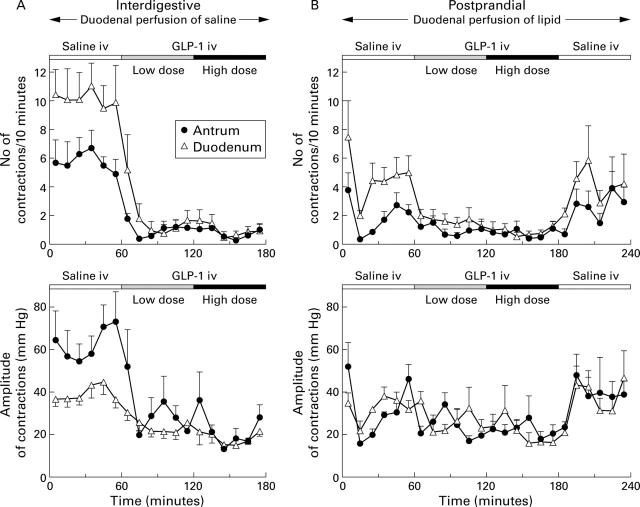
Contraction frequencies (top) and amplitudes (bottom) in the antrum and duodenum in response to intravenous infusions of saline, and GLP-1(7-36)amide 0.4 and 1.2 pmol/kg/min during concomitant duodenal perfusion of saline (A) or lipid 2.5 kcal/min (B) in 11 healthy volunteers (mean (SEM)). For statistical analysis, see table 2.
Figure 3 .
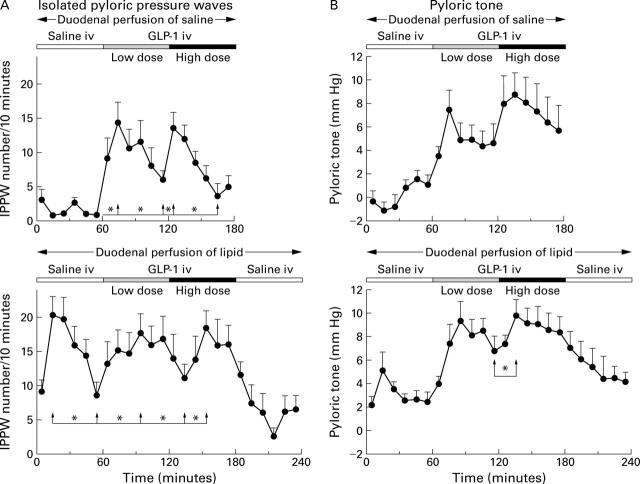
Isolated pyloric pressure waves (IPPW) (A) and pyloric tone (B) in response to intravenous infusions of saline, and GLP-1(7-36)amide 0.4 and 1.2 pmol/kg/min during concomitant duodenal perfusion of saline (top) or lipid 2.5 kcal/min (bottom) in 11 healthy volunteers (mean (SEM)). *p<0.05 for comparison of times indicated by the arrows (paired t test). For further statistical analysis, see table 3.
Figure 4 .
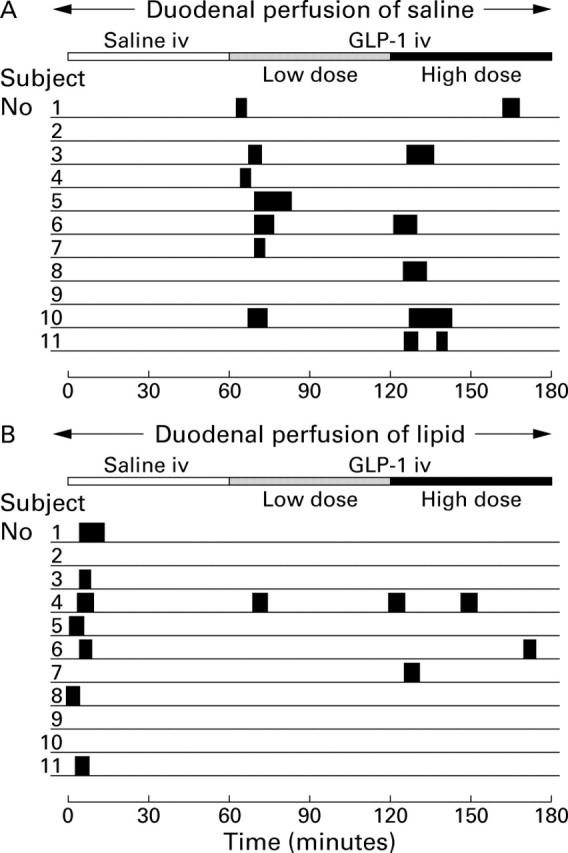
Occurrence of duodenal phase III-like activity with duodenal perfusion of saline (A) or lipid 2.5 kcal/min (B) in 11 healthy volunteers. In seven of 11 subjects, an activity front was seen within 10 minutes after the start of the low dose infusion of GLP-1 in the interdigestive state or duodenal lipid perfusion in the postprandial studies, respectively. The length of the solid bars represents the length of contraction burst.
Figure 5 .
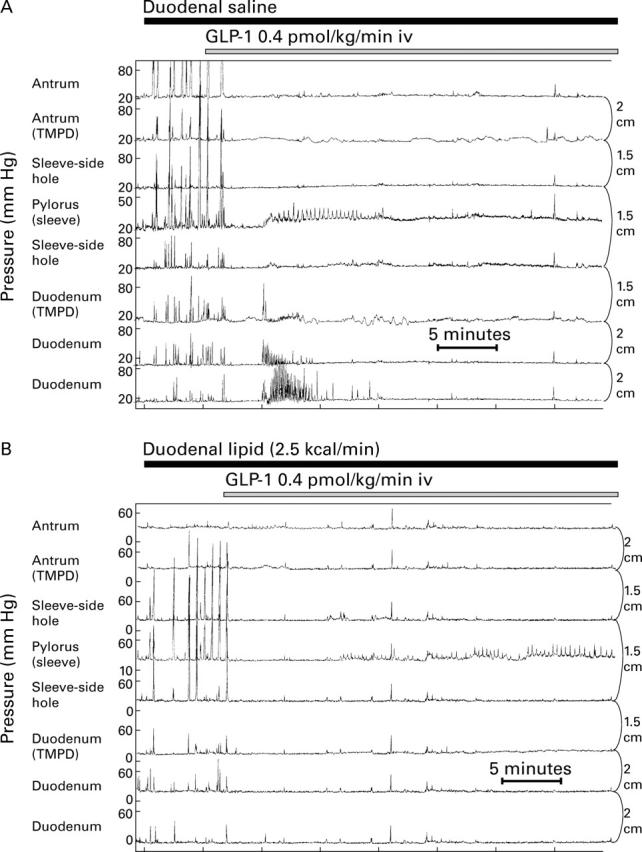
Manometric tracings showing the effects of intravenous infusion of GLP-1 0.4 pmol/kg/min during duodenal perfusion of saline (A) and lipid 2.5 kcal/min (B). In the interdigestive state (A), GLP-1 immediately inhibited antro-duodenal motility and induced a sustained increase in basal pyloric pressure with concomitant short lasting stimulation of IPPWs. During duodenal lipid perfusion (B), antral and duodenal contractility were completely abolished by GLP-1, and basal pyloric pressure further increased in addition to the effect of lipid alone, paralleled by stimulation of IPPWs.
Figure 6 .
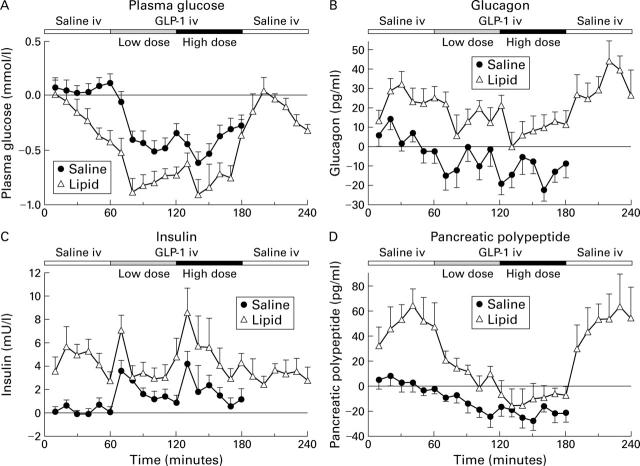
Effects of intravenous infusions of saline, and GLP-1(7-36)amide 0.4 and 1.2 pmol/kg/min on plasma glucose (A) and immunoreactivities of glucagon (B), insulin (C), and pancreatic polypeptide (D) during concomitant duodenal perfusion of saline or lipid 2.5 kcal/min in 11 healthy volunteers. Mean (SEM) of incremental values over basal. For statistical analysis, see table 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allescher H. D., Daniel E. E., Dent J., Fox J. E., Kostolanska F. Extrinsic and intrinsic neural control of pyloric sphincter pressure in the dog. J Physiol. 1988 Jul;401:17–38. doi: 10.1113/jphysiol.1988.sp017149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allescher H. D., Tougas G., Vergara P., Lu S., Daniel E. E. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Physiol. 1992 Apr;262(4 Pt 1):G695–G702. doi: 10.1152/ajpgi.1992.262.4.G695. [DOI] [PubMed] [Google Scholar]
- Anvari M., Dent J., Malbert C., Jamieson G. G. Mechanics of pulsatile transpyloric flow in the pig. J Physiol. 1995 Oct 1;488(Pt 1):193–202. doi: 10.1113/jphysiol.1995.sp020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvari M., Paterson C. A., Daniel E. E., McDonald T. J. Effects of GLP-1 on gastric emptying, antropyloric motility, and transpyloric flow in response to a nonnutrient liquid. Dig Dis Sci. 1998 Jun;43(6):1133–1140. doi: 10.1023/a:1018863716749. [DOI] [PubMed] [Google Scholar]
- Anvari M., Paterson C. A., Daniel E. E. Role of nitric oxide mechanisms in control of pyloric motility and transpyloric flow of liquids in conscious dogs. Dig Dis Sci. 1998 Mar;43(3):506–512. doi: 10.1023/a:1018898621465. [DOI] [PubMed] [Google Scholar]
- Barnett J. L., Owyang C. Serum glucose concentration as a modulator of interdigestive gastric motility. Gastroenterology. 1988 Mar;94(3):739–744. doi: 10.1016/0016-5085(88)90248-x. [DOI] [PubMed] [Google Scholar]
- Brener W., Hendrix T. R., McHugh P. R. Regulation of the gastric emptying of glucose. Gastroenterology. 1983 Jul;85(1):76–82. [PubMed] [Google Scholar]
- Byrne M. M., Pluntke K., Wank U., Schirra J., Arnold R., Göke B., Katschinski M. Inhibitory effects of hyperglycaemia on fed jejunal motility: potential role of hyperinsulinaemia. Eur J Clin Invest. 1998 Jan;28(1):72–78. doi: 10.1046/j.1365-2362.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Malagelada J. R., Brown M. L., Becker G., Zinsmeister A. R. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985 Nov;249(5 Pt 1):G580–G585. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Malagelada J. R., Stanghellini V., Zinsmeister A. R., Kao P. C., Li C. H. Dose-related effects of synthetic human beta-endorphin and naloxone on fed gastrointestinal motility. Am J Physiol. 1986 Jul;251(1 Pt 1):G147–G154. doi: 10.1152/ajpgi.1986.251.1.G147. [DOI] [PubMed] [Google Scholar]
- Dupre J., Behme M. T., Hramiak I. M., McFarlane P., Williamson M. P., Zabel P., McDonald T. J. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes. 1995 Jun;44(6):626–630. doi: 10.2337/diab.44.6.626. [DOI] [PubMed] [Google Scholar]
- Ebert R., Frerichs H., Creutzfeldt W. Impaired feedback control of fat induced gastric inhibitory polypeptide (GIP) secretion by insulin in obesity and glucose intolerance. Eur J Clin Invest. 1979 Apr;9(2 Pt 1):129–135. doi: 10.1111/j.1365-2362.1979.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Elahi D., McAloon-Dyke M., Fukagawa N. K., Meneilly G. S., Sclater A. L., Minaker K. L., Habener J. F., Andersen D. K. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept. 1994 Apr 14;51(1):63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Eliasson B., Björnsson E., Urbanavicius V., Andersson H., Fowelin J., Attvall S., Abrahamsson H., Smith U. Hyperinsulinaemia impairs gastrointestinal motility and slows carbohydrate absorption. Diabetologia. 1995 Jan;38(1):79–85. doi: 10.1007/BF02369356. [DOI] [PubMed] [Google Scholar]
- Fehmann H. C., Göke R., Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995 Jun;16(3):390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- Fehmann H. C., Hering B. J., Wolf M. J., Brandhorst H., Brandhorst D., Bretzel R. G., Federlin K., Göke B. The effects of glucagon-like peptide-I (GLP-I) on hormone secretion from isolated human pancreatic islets. Pancreas. 1995 Aug;11(2):196–200. doi: 10.1097/00006676-199508000-00014. [DOI] [PubMed] [Google Scholar]
- Fone D. R., Horowitz M., Dent J., Read N. W., Heddle R. Pyloric motor response to intraduodenal dextrose involves muscarinic mechanisms. Gastroenterology. 1989 Jul;97(1):83–90. doi: 10.1016/0016-5085(89)91419-4. [DOI] [PubMed] [Google Scholar]
- Fone D. R., Horowitz M., Maddox A., Akkermans L. M., Read N. W., Dent J. Gastroduodenal motility during the delayed gastric emptying induced by cold stress. Gastroenterology. 1990 May;98(5 Pt 1):1155–1161. doi: 10.1016/0016-5085(90)90328-x. [DOI] [PubMed] [Google Scholar]
- Fraser R., Fone D., Horowitz M., Dent J. Cholecystokinin octapeptide stimulates phasic and tonic pyloric motility in healthy humans. Gut. 1993 Jan;34(1):33–37. doi: 10.1136/gut.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R., Horowitz M., Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991 May;32(5):475–478. doi: 10.1136/gut.32.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R., Horowitz M., Maddox A., Dent J. Dual effects of cisapride on gastric emptying and antropyloroduodenal motility. Am J Physiol. 1993 Feb;264(2 Pt 1):G195–G201. doi: 10.1152/ajpgi.1993.264.2.G195. [DOI] [PubMed] [Google Scholar]
- Gutniak M., Orskov C., Holst J. J., Ahrén B., Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992 May 14;326(20):1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- Gutzwiller J. P., Göke B., Drewe J., Hildebrand P., Ketterer S., Handschin D., Winterhalder R., Conen D., Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999 Jan;44(1):81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R., Fehmann H. C., Göke B. Glucagon-like peptide-1(7-36) amide is a new incretin/enterogastrone candidate. Eur J Clin Invest. 1991 Apr;21(2):135–144. doi: 10.1111/j.1365-2362.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Göke R., Larsen P. J., Mikkelsen J. D., Sheikh S. P. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995 Nov 1;7(11):2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Heddle R., Dent J., Read N. W., Houghton L. A., Toouli J., Horowitz M., Maddern G. J., Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol. 1988 May;254(5 Pt 1):G671–G679. doi: 10.1152/ajpgi.1988.254.5.G671. [DOI] [PubMed] [Google Scholar]
- Heddle R., Fone D., Dent J., Horowitz M. Stimulation of pyloric motility by intraduodenal dextrose in normal subjects. Gut. 1988 Oct;29(10):1349–1357. doi: 10.1136/gut.29.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddle R., Miedema B. W., Kelly K. A. Integration of canine proximal gastric, antral, pyloric, and proximal duodenal motility during fasting and after a liquid meal. Dig Dis Sci. 1993 May;38(5):856–869. doi: 10.1007/BF01295912. [DOI] [PubMed] [Google Scholar]
- Holle G. E., Hahn D., Forth W. Innervation of pylorus in control of motility and gastric emptying. Am J Physiol. 1992 Aug;263(2 Pt 1):G161–G168. doi: 10.1152/ajpgi.1992.263.2.G161. [DOI] [PubMed] [Google Scholar]
- Houghton L. A., Read N. W., Heddle R., Maddern G. J., Downton J., Toouli J., Dent J. Motor activity of the gastric antrum, pylorus, and duodenum under fasted conditions and after a liquid meal. Gastroenterology. 1988 Jun;94(6):1276–1284. doi: 10.1016/0016-5085(88)90664-6. [DOI] [PubMed] [Google Scholar]
- Imeryüz N., Yeğen B. C., Bozkurt A., Coşkun T., Villanueva-Peñacarrillo M. L., Ulusoy N. B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997 Oct;273(4 Pt 1):G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Katschinski M., Dahmen G., Reinshagen M., Beglinger C., Koop H., Nustede R., Adler G. Cephalic stimulation of gastrointestinal secretory and motor responses in humans. Gastroenterology. 1992 Aug;103(2):383–391. doi: 10.1016/0016-5085(92)90825-j. [DOI] [PubMed] [Google Scholar]
- Katschinski M., Schirra J., Begliner C., Langbein S., Wank U., D'Amato M., Arnold R. Intestinal phase of human antro-pyloro-duodenal motility: cholinergic and CCK-mediated regulation. Eur J Clin Invest. 1996 Jul;26(7):574–583. doi: 10.1046/j.1365-2362.1996.1790522.x. [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987 Dec 5;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Doty J. E., Reedy T. J., Meyer J. H. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol. 1989 Feb;256(2 Pt 1):G404–G411. doi: 10.1152/ajpgi.1989.256.2.G404. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Doty J. E., Reedy T. J., Meyer J. H. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990 Dec;259(6 Pt 1):G1031–G1036. doi: 10.1152/ajpgi.1990.259.6.G1031. [DOI] [PubMed] [Google Scholar]
- Malbert C. H., Mathis C. Antropyloric modulation of transpyloric flow of liquids in pigs. Gastroenterology. 1994 Jul;107(1):37–46. doi: 10.1016/0016-5085(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Nauck M. A., Bartels E., Orskov C., Ebert R., Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993 Apr;76(4):912–917. doi: 10.1210/jcem.76.4.8473405. [DOI] [PubMed] [Google Scholar]
- Nauck M. A., Heimesaat M. M., Orskov C., Holst J. J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993 Jan;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J., Katschinski M., Weidmann C., Schäfer T., Wank U., Arnold R., Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996 Jan 1;97(1):92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J., Kuwert P., Wank U., Leicht P., Arnold R., Göke B., Katschinski M. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Physicians. 1997 Jan;109(1):84–97. [PubMed] [Google Scholar]
- Schirra J., Leicht P., Hildebrand P., Beglinger C., Arnold R., Göke B., Katschinski M. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. J Endocrinol. 1998 Jan;156(1):177–186. doi: 10.1677/joe.0.1560177. [DOI] [PubMed] [Google Scholar]
- Schirra J., Sturm K., Leicht P., Arnold R., Göke B., Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998 Apr 1;101(7):1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. W. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983 Dec;85(6):1411–1425. [PubMed] [Google Scholar]
- Stanghellini V., Malagelada J. R., Zinsmeister A. R., Go V. L., Kao P. C. Stress-induced gastroduodenal motor disturbances in humans: possible humoral mechanisms. Gastroenterology. 1983 Jul;85(1):83–91. [PubMed] [Google Scholar]
- Takahashi H., Manaka H., Suda K., Fukase N., Sekikawa A., Eguchi H., Tominaga M., Sasaki H. Hyperglycaemia but not hyperinsulinaemia prevents the secretion of glucagon-like peptide-1 (7-36 amide) stimulated by fat ingestion. Scand J Clin Lab Invest. 1991 Oct;51(6):499–507. doi: 10.3109/00365519109104558. [DOI] [PubMed] [Google Scholar]
- Taylor I., Duthie H. L., Cumberland D. C., Smallwood R. Glucagon and the colon. Gut. 1975 Dec;16(12):973–978. doi: 10.1136/gut.16.12.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. G., Richelson E., Malagelada J. R. Perturbation of gastric emptying and duodenal motility through the central nervous system. Gastroenterology. 1982 Dec;83(6):1200–1206. [PubMed] [Google Scholar]
- Tougas G., Anvari M., Dent J., Somers S., Richards D., Stevenson G. W. Relation of pyloric motility to pyloric opening and closure in healthy subjects. Gut. 1992 Apr;33(4):466–471. doi: 10.1136/gut.33.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton M. D., O'Shea D., Gunn I., Beak S. A., Edwards C. M., Meeran K., Choi S. J., Taylor G. M., Heath M. M., Lambert P. D. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996 Jan 4;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Wettergren A., Schjoldager B., Mortensen P. E., Myhre J., Christiansen J., Holst J. J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993 Apr;38(4):665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]