Abstract
BACKGROUND—Cell death by apoptosis seems to be an important mechanism for translocation to the cell surface of a variety of intracellular components capable of inducing autoantibody production. AIMS—To identify the cellular location of antigen (Ag)-antineutrophil cytoplasmic antibodies (ANCA) in non-apoptotic human neutrophils, and to assess if ANCA associated with ulcerative colitis reacts with neutrophil antigen(s) during neutrophil apoptosis. The cellular distribution of Ag-ANCA in apoptotic neutrophils was also investigated. METHODS—Sera from 18 ulcerative colitis patients known to be positive for perinuclear IgG-ANCA (titre ⩾1/320), as assessed by indirect immunofluorescence (IIF), were analysed by immunofluorescent confocal laser scanning microscopy. ANCA were identified with fluorescein isothiocyanate (FITC) and tetramethylrhodamine isothiocyanate (TRITC) in non-apoptotic and apoptotic neutrophils, respectively. Apoptotic and non-apoptotic DNA was labelled with FITC and propidium iodide, respectively. Cycloheximide was added to polymorphonuclear leucocyte culture to induce apoptosis. RESULTS—Three patterns of scanning laser immunofluorescence microscopy in non-apoptotic neutrophils were observed with respect to cellular ulcerative colitis associated ANCA distribution: (1) diffuse nuclear localisation (16.7%); (2) nuclear localisation in the nuclear periphery (50%); and (3) mixed nuclear and cytoplasmic localisation (33.4%). In all sera ANCA fluorescence colocalised almost completely with apoptotic DNA, with persistence of a diffuse and intense fluorescence. No significant changes in ANCA titres were found in non-apoptotic neutrophils. CONCLUSIONS—The antigen(s) of ANCA associated with ulcerative colitis seems to be localised in most cases in the neutrophil nucleus. The almost identical colocalisation of ANCA and apoptotic cleaved DNA suggests that intracellular DNA redistribution during neutrophil apoptosis may play a role in antigen exposure to the immune system and ANCA production in ulcerative colitis. Keywords: antineutrophil cytoplasmic antibody; antigen; ulcerative colitis; apoptosis; humoral immunity; immunofluorescent laser confocal microscopy
Full Text
The Full Text of this article is available as a PDF (191.8 KB).
Figure 1 .
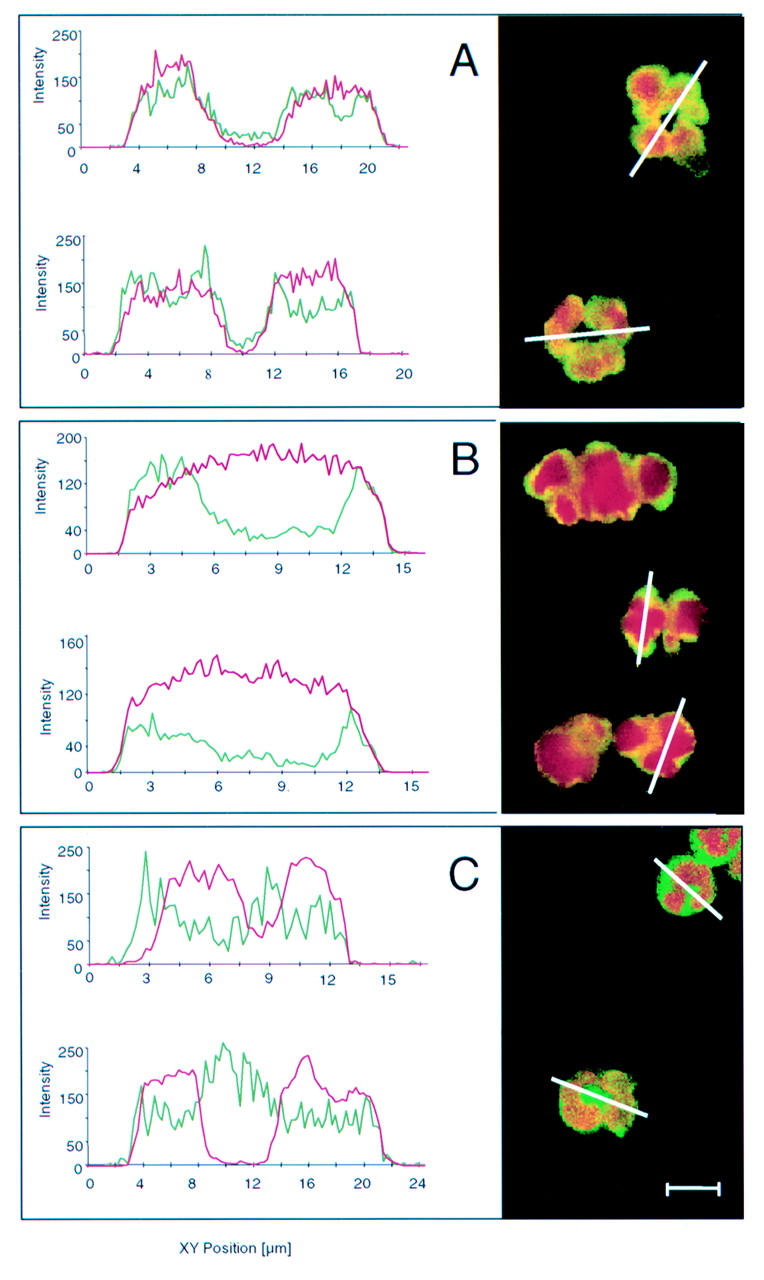
Confocal laser scanning microscopy view of non-apoptotic ethanol fixed neutrophils. Antineutrophil cytoplasmic antibodies (ANCA) were detected with fluorescein isothiocyanate (FITC) conjugated secondary antibodies that produce a green signal at a wavelength of 500-560 nm. DNA was visualised with propidium iodide which gives a red signal at wavelengths longer than 590 nm. Three different patterns of ANCA localisation may be observed. (A) A diffuse nuclear ANCA associated ulcerative colitis (UC-ANCA) staining pattern, as demonstrated by yellow staining in the whole nuclear area as a result of superimposition of the two fluorescence signals. Complete overlay of the profile of staining intensity obtained by channel 1 (green profile, ANCA) and that obtained by channel 2 (red profile, DNA) may be observed. (B) Peripheral nuclear staining pattern, as demonstrated by a yellow rim-like ANCA fluorescence in the inner side of the nuclear periphery, overlapping with DNA. Yellow staining may be observed in the peripheral nuclear area as a result of superimposition of the two fluorescence signals. The profile obtained by channel 1 (green profile, ANCA) mainly localises in the inner side of the nucleus (channel 2, red profile, DNA) periphery, as demonstrated by two peaks of greater intensity of ANCA staining in this location. (C) Mixed nuclear and cytoplasmic staining pattern. The green staining of the fluorescence signal of ANCA, non-superimposed with DNA, may be observed. The profile obtained by channel 1 (green profile, ANCA) is partly localised in the nucleus (channel 2, red profile, DNA) but overwhelms the DNA fluorescence boundary. Bar=10 µm.
Figure 2 .
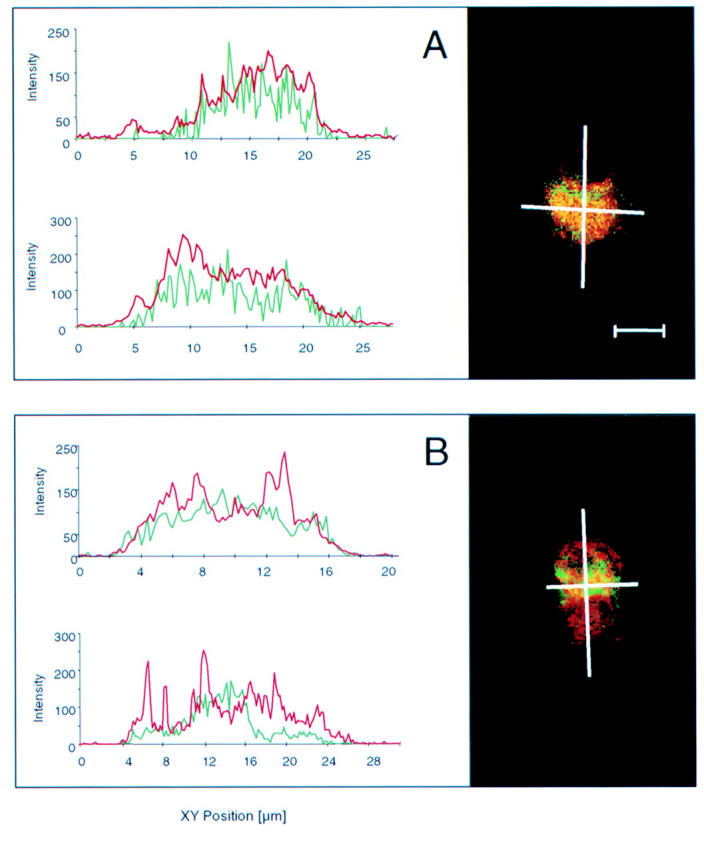
Confocal laser scanning microscopy of apoptotic ethanol fixed neutrophils. Antineutrophil cytoplasmic antibodies (ANCA) were detected with tetramethylrhodamine isothiocyanate (TRITC) which produces a red signal at wavelengths longer than 590 nm. Apoptotic DNA was visualised with TUNEL (TdT mediated FITC-dUTP nick end labelling), an in situ programmed cell death labelling method based on detection of DNA strand breaks labelled with fluorescein isothiocyanate (FITC) which gives a green signal at wavelengths of 500-560 nm. (A) With most sera, ANCA staining showed diffuse colocalisation with apoptotic cleaved DNA (oligonucleosomes and nucleosomes). Cross over fluorescence between FITC (cleaved DNA) and TRITC (ANCA) is shown by yellow staining. Almost complete superimposition of the profile of staining intensity obtained by channel 1 (red profile, ANCA) and that obtained by channel 2 (green profile, cleaved DNA) may be observed. (B) With three sera the ANCA staining intensity (red signal) was higher than that of apoptotic DNA (green signal) in some areas of the apoptotic neutrophils. These areas morphologically correspond to apoptotic blebs beyond the cytoplasmic membrane. The ANCA fluorescence profile (green profile) partly superimposed with the DNA profile (red profile) but exceeded the boundary of the apoptotic DNA. Bar=10 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYUM A. SEPARATION OF WHITE BLOOD CELLS. Nature. 1964 Nov 21;204:793–794. doi: 10.1038/204793a0. [DOI] [PubMed] [Google Scholar]
- Billing P., Tahir S., Calfin B., Gagne G., Cobb L., Targan S., Vidrich A. Nuclear localization of the antigen detected by ulcerative colitis-associated perinuclear antineutrophil cytoplasmic antibodies. Am J Pathol. 1995 Oct;147(4):979–987. [PMC free article] [PubMed] [Google Scholar]
- Burlingame R. W., Boey M. L., Starkebaum G., Rubin R. L. The central role of chromatin in autoimmune responses to histones and DNA in systemic lupus erythematosus. J Clin Invest. 1994 Jul;94(1):184–192. doi: 10.1172/JCI117305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D. D., Krishnan M. R., Swindle J. T., Marion T. N. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993 Aug 1;151(3):1614–1626. [PubMed] [Google Scholar]
- Ellerbroek P. M., Oudkerk Pool M., Ridwan B. U., Dolman K. M., von Blomberg B. M., von dem Borne A. E., Meuwissen S. G., Goldschmeding R. Neutrophil cytoplasmic antibodies (p-ANCA) in ulcerative colitis. J Clin Pathol. 1994 Mar;47(3):257–262. doi: 10.1136/jcp.47.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve M., Mallolas J., Klaassen J., Abad-Lacruz A., González-Huix F., Cabré E., Fernández-Bañares F., Bertrán X., Condom E., Martí-Ragué J. Antineutrophil cytoplasmic antibodies in sera from colectomised ulcerative colitis patients and its relation to the presence of pouchitis. Gut. 1996 Jun;38(6):894–898. doi: 10.1136/gut.38.6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998 Jul;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan H. M., Bredy B., Brady H. R., Hébert M. J., Slayter H. S., Xu Y., Rauch J., Shia M. A., Koh J. S., Levine J. S. Antineutrophil cytoplasmic autoantibodies interact with primary granule constituents on the surface of apoptotic neutrophils in the absence of neutrophil priming. J Exp Med. 1996 Dec 1;184(6):2231–2241. doi: 10.1084/jem.184.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Vecchi M., Rizzello F., Ferretti M., Calabresi C., Venturi A., Bianchi M. B., Brignola C., Sinico R. A., De Franchis R. Lack of effect of antineutrophil cytoplasmic antibodies associated with ulcerative colitis on superoxide anion production from neutrophils. Gut. 1997 Jan;40(1):102–104. doi: 10.1136/gut.40.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H. Antibodies to DNA. N Engl J Med. 1998 May 7;338(19):1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- Haslett C. Granulocyte apoptosis and inflammatory disease. Br Med Bull. 1997;53(3):669–683. doi: 10.1093/oxfordjournals.bmb.a011638. [DOI] [PubMed] [Google Scholar]
- Lennard-Jones J. E. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Smith R. N., Preffer F. I., Bhan A. K. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997 Nov 17;186(10):1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudkerk Pool M., Ellerbroek P. M., Ridwan B. U., Goldschmeding R., von Blomberg B. M., Peña A. S., Dolman K. M., Bril H., Dekker W., Nauta J. J. Serum antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease are mainly associated with ulcerative colitis. A correlation study between perinuclear antineutrophil cytoplasmic autoantibodies and clinical parameters, medical, and surgical treatment. Gut. 1993 Jan;34(1):46–50. doi: 10.1136/gut.34.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C. M., Glasser L., Tischler M. E., Wyckoff D., Cromey D., Fiederlein R., Bohnert O. Programmed cell death of the normal human neutrophil: an in vitro model of senescence. Microsc Res Tech. 1994 Jul 1;28(4):327–344. doi: 10.1002/jemt.1070280408. [DOI] [PubMed] [Google Scholar]
- Porges A. J., Redecha P. B., Kimberly W. T., Csernok E., Gross W. L., Kimberly R. P. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994 Aug 1;153(3):1271–1280. [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobajima J., Ozaki S., Osakada F., Uesugi H., Shirakawa H., Yoshida M., Nakao K. Novel autoantigens of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) in ulcerative colitis: non-histone chromosomal proteins, HMG1 and HMG2. Clin Exp Immunol. 1997 Jan;107(1):135–140. doi: 10.1046/j.1365-2249.1997.d01-907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga R., Abdou N. I. Anti-(DNA-histone) antibodies in active lupus nephritis. J Rheumatol. 1996 Feb;23(2):279–284. [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax W. J., Kramers C., van Bruggen M. C., Berden J. H. Apoptosis, nucleosomes, and nephritis in systemic lupus erythematosus. Kidney Int. 1995 Sep;48(3):666–673. doi: 10.1038/ki.1995.336. [DOI] [PubMed] [Google Scholar]
- Terjung B., Herzog V., Worman H. J., Gestmann I., Bauer C., Sauerbruch T., Spengler U. Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders colocalize with nuclear lamina proteins. Hepatology. 1998 Aug;28(2):332–340. doi: 10.1002/hep.510280207. [DOI] [PubMed] [Google Scholar]
- Tsuchida H., Takeda Y., Takei H., Shinzawa H., Takahashi T., Sendo F. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-alpha or cycloheximide. J Immunol. 1995 Mar 1;154(5):2403–2412. [PubMed] [Google Scholar]
- Walmsley R. S., Zhao M. H., Hamilton M. I., Brownlee A., Chapman P., Pounder R. E., Wakefield A. J., Lockwood C. M. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997 Jan;40(1):105–109. doi: 10.1136/gut.40.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino S. J., Singh M. K., Bass J., Picker L. J. Immunodetection of histone epitopes correlates with early stages of apoptosis in activated human peripheral T lymphocytes. Am J Pathol. 1996 Aug;149(2):653–663. [PMC free article] [PubMed] [Google Scholar]


