Full Text
The Full Text of this article is available as a PDF (234.5 KB).
Figure 1 .

Mismatch repair. A mispaired base is recognised by the hMSH2/GTBP complex while an insertion/deletion loop is recognised by the hMSH2/hMSH3 complex. MutL related proteins (hMLH1/hPMS2 and hMLH1/hPMS1 complexes) then interact with the MutS related proteins that are already bound to the mispaired bases. (The hMSH2/GTBP complex may also support the repair of insertion/deletion loops).
Figure 2 .
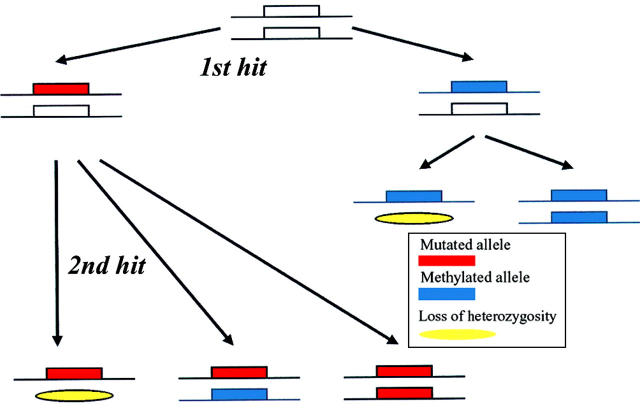
Methylation and Knudson's two hit hypothesis. It has been proposed that epigenetic mechanisms, such as hypermethylation of the promoter region, should be included in the two hit hypothesis for inactivation of tumour suppressor genes. It was suggested that the first hit may be a mutation in the DNA sequence or promoter methylation. The second inactivating hit may be either loss of heterozygosity or a further mutational or methylating event in the second allele.
Figure 3 .
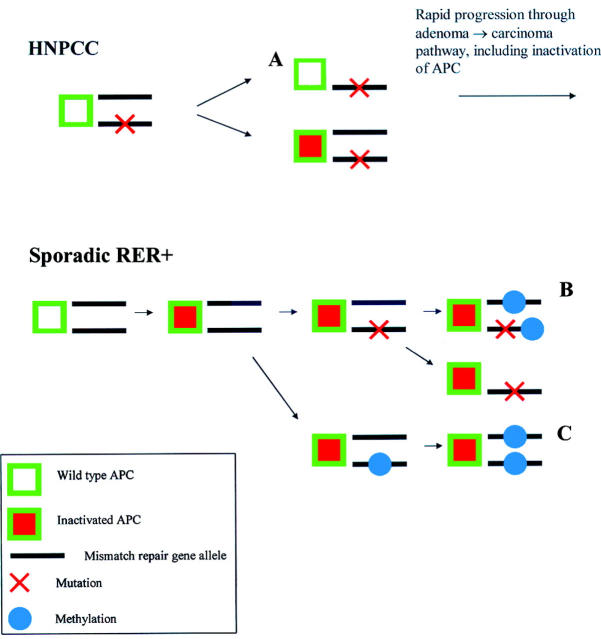
Model for adenoma→carcinoma pathway in HNPCC versus sporadic RER+ colorectal cancers. In HNPCC, a germline mutation is present in every cell, and only one further event (usually loss of heterozygosity (LOH)) is required to inactivate a mismatch repair gene. This may occur at an early stage (A), before inactivation of APC, and result in rapid progression through the adenoma→carcinoma pathway. In contrast, inactivation of a mismatch repair gene in sporadic RER+ cancers is likely to be a late event, after inactivation of APC. Although inactivation may be due to a somatic mutation (with LOH), hypermethylation of the hMLH1 promoter region is the commonest cause of inactivation of mismatch repair genes in these cancers, and is usually a biallelic event (B, C).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Aaltonen L. A., Peltomäki P., Mecklin J. P., Järvinen H., Jass J. R., Green J. S., Lynch H. T., Watson P., Tallqvist G., Juhola M. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994 Apr 1;54(7):1645–1648. [PubMed] [Google Scholar]
- Aaltonen L. A., Salovaara R., Kristo P., Canzian F., Hemminki A., Peltomäki P., Chadwick R. B., Käriäinen H., Eskelinen M., Järvinen H. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998 May 21;338(21):1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- Ahuja N., Mohan A. L., Li Q., Stolker J. M., Herman J. G., Hamilton S. R., Baylin S. B., Issa J. P. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997 Aug 15;57(16):3370–3374. [PubMed] [Google Scholar]
- Akiyama Y., Sato H., Yamada T., Nagasaki H., Tsuchiya A., Abe R., Yuasa Y. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997 Sep 15;57(18):3920–3923. [PubMed] [Google Scholar]
- Alani E. The Saccharomyces cerevisiae Msh2 and Msh6 proteins form a complex that specifically binds to duplex oligonucleotides containing mismatched DNA base pairs. Mol Cell Biol. 1996 Oct;16(10):5604–5615. doi: 10.1128/mcb.16.10.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzimanoglou I. I., Gilbert F., Barber H. R. Microsatellite instability in human solid tumors. Cancer. 1998 May 15;82(10):1808–1820. doi: 10.1002/(sici)1097-0142(19980515)82:10<1808::aid-cncr2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. 1998 Runme Shaw Memorial Lecture: somatic evolution of cancer. Ann Acad Med Singapore. 1999 May;28(3):323–329. [PubMed] [Google Scholar]
- Boland C. R., Thibodeau S. N., Hamilton S. R., Sidransky D., Eshleman J. R., Burt R. W., Meltzer S. J., Rodriguez-Bigas M. A., Fodde R., Ranzani G. N. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998 Nov 15;58(22):5248–5257. [PubMed] [Google Scholar]
- Boland C. R., Troncale F. J. Familial colonic cancer without antecedent polyposis. Ann Intern Med. 1984 May;100(5):700–701. doi: 10.7326/0003-4819-100-5-700. [DOI] [PubMed] [Google Scholar]
- Branch P., Bicknell D. C., Rowan A., Bodmer W. F., Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995 Mar;9(3):231–232. doi: 10.1038/ng0395-231. [DOI] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Børresen A. L., Lothe R. A., Meling G. I., Lystad S., Morrison P., Lipford J., Kane M. F., Rognum T. O., Kolodner R. D. Somatic mutations in the hMSH2 gene in microsatellite unstable colorectal carcinomas. Hum Mol Genet. 1995 Nov;4(11):2065–2072. doi: 10.1093/hmg/4.11.2065. [DOI] [PubMed] [Google Scholar]
- Cahill D. P., Lengauer C., Yu J., Riggins G. J., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998 Mar 19;392(6673):300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Cameron E. E., Bachman K. E., Myöhänen S., Herman J. G., Baylin S. B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999 Jan;21(1):103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Harrison J., Molloy P. L. Sp1 binding is inhibited by (m)Cp(m)CpG methylation. Gene. 1997 Aug 11;195(1):67–71. doi: 10.1016/s0378-1119(97)00164-9. [DOI] [PubMed] [Google Scholar]
- Cunningham J. M., Christensen E. R., Tester D. J., Kim C. Y., Roche P. C., Burgart L. J., Thibodeau S. N. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998 Aug 1;58(15):3455–3460. [PubMed] [Google Scholar]
- Drotschmann K., Aronshtam A., Fritz H. J., Marinus M. G. The Escherichia coli MutL protein stimulates binding of Vsr and MutS to heteroduplex DNA. Nucleic Acids Res. 1998 Feb 15;26(4):948–953. doi: 10.1093/nar/26.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond J. T., Li G. M., Longley M. J., Modrich P. Isolation of an hMSH2-p160 heterodimer that restores DNA mismatch repair to tumor cells. Science. 1995 Jun 30;268(5219):1909–1912. doi: 10.1126/science.7604264. [DOI] [PubMed] [Google Scholar]
- Dunlop M. G., Farrington S. M., Carothers A. D., Wyllie A. H., Sharp L., Burn J., Liu B., Kinzler K. W., Vogelstein B. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997 Jan;6(1):105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- Eshleman J. R., Casey G., Kochera M. E., Sedwick W. D., Swinler S. E., Veigl M. L., Willson J. K., Schwartz S., Markowitz S. D. Chromosome number and structure both are markedly stable in RER colorectal cancers and are not destabilized by mutation of p53. Oncogene. 1998 Aug 13;17(6):719–725. doi: 10.1038/sj.onc.1201986. [DOI] [PubMed] [Google Scholar]
- Eshleman J. R., Markowitz S. D. Mismatch repair defects in human carcinogenesis. Hum Mol Genet. 1996;5(Spec No):1489–1494. doi: 10.1093/hmg/5.supplement_1.1489. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H., Kolodner R. D. The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12404–12409. doi: 10.1073/pnas.95.21.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Enomoto T., Yoshino K., Nomura T., Buzard G. S., Inoue M., Okudaira Y. Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer. 1995 Dec 20;64(6):361–366. doi: 10.1002/ijc.2910640602. [DOI] [PubMed] [Google Scholar]
- Genschel J., Littman S. J., Drummond J. T., Modrich P. Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J Biol Chem. 1998 Jul 31;273(31):19895–19901. doi: 10.1074/jbc.273.31.19895. [DOI] [PubMed] [Google Scholar]
- Greene C. N., Jinks-Robertson S. Frameshift intermediates in homopolymer runs are removed efficiently by yeast mismatch repair proteins. Mol Cell Biol. 1997 May;17(5):2844–2850. doi: 10.1128/mcb.17.5.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habraken Y., Sung P., Prakash L., Prakash S. Binding of insertion/deletion DNA mismatches by the heterodimer of yeast mismatch repair proteins MSH2 and MSH3. Curr Biol. 1996 Sep 1;6(9):1185–1187. doi: 10.1016/s0960-9822(02)70686-6. [DOI] [PubMed] [Google Scholar]
- Habraken Y., Sung P., Prakash L., Prakash S. Enhancement of MSH2-MSH3-mediated mismatch recognition by the yeast MLH1-PMS1 complex. Curr Biol. 1997 Oct 1;7(10):790–793. doi: 10.1016/s0960-9822(06)00337-x. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Liu B., Parsons R. E., Papadopoulos N., Jen J., Powell S. M., Krush A. J., Berk T., Cohen Z., Tetu B. The molecular basis of Turcot's syndrome. N Engl J Med. 1995 Mar 30;332(13):839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Herman J. G., Umar A., Polyak K., Graff J. R., Ahuja N., Issa J. P., Markowitz S., Willson J. K., Hamilton S. R., Kinzler K. W. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang J. M., Cottu P. H., Thuille B., Salmon R. J., Thomas G., Hamelin R. BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res. 1997 Jan 15;57(2):300–303. [PubMed] [Google Scholar]
- Homfray T. F., Cottrell S. E., Ilyas M., Rowan A., Talbot I. C., Bodmer W. F., Tomlinson I. P. Defects in mismatch repair occur after APC mutations in the pathogenesis of sporadic colorectal tumours. Hum Mutat. 1998;11(2):114–120. doi: 10.1002/(SICI)1098-1004(1998)11:2<114::AID-HUMU3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Huang J., Papadopoulos N., McKinley A. J., Farrington S. M., Curtis L. J., Wyllie A. H., Zheng S., Willson J. K., Markowitz S. D., Morin P. APC mutations in colorectal tumors with mismatch repair deficiency. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jass J. R. Colorectal adenomas in surgical specimens from subjects with hereditary non-polyposis colorectal cancer. Histopathology. 1995 Sep;27(3):263–267. doi: 10.1111/j.1365-2559.1995.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Cottier D. S., Jeevaratnam P., Pokos V., Holdaway K. M., Bowden M. L., Van de Water N. S., Browett P. J. Diagnostic use of microsatellite instability in hereditary non-polyposis colorectal cancer. Lancet. 1995 Nov 4;346(8984):1200–1201. doi: 10.1016/s0140-6736(95)92902-9. [DOI] [PubMed] [Google Scholar]
- Jass J. R. Diagnosis of hereditary non-polyposis colorectal cancer. Histopathology. 1998 Jun;32(6):491–497. doi: 10.1046/j.1365-2559.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Do K. A., Simms L. A., Iino H., Wynter C., Pillay S. P., Searle J., Radford-Smith G., Young J., Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998 May;42(5):673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R., Edgar S. Unicryptal loss of heterozygosity in hereditary non-polyposis colorectal cancer. Pathology. 1994 Oct;26(4):414–417. doi: 10.1080/00313029400169102. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Smyrk T. C., Stewart S. M., Lane M. R., Lanspa S. J., Lynch H. T. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994 Jul-Aug;14(4B):1631–1634. [PubMed] [Google Scholar]
- Jones P. A., Laird P. W. Cancer epigenetics comes of age. Nat Genet. 1999 Feb;21(2):163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- Kane M. F., Loda M., Gaida G. M., Lipman J., Mishra R., Goldman H., Jessup J. M., Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997 Mar 1;57(5):808–811. [PubMed] [Google Scholar]
- Katabuchi H., van Rees B., Lambers A. R., Ronnett B. M., Blazes M. S., Leach F. S., Cho K. R., Hedrick L. Mutations in DNA mismatch repair genes are not responsible for microsatellite instability in most sporadic endometrial carcinomas. Cancer Res. 1995 Dec 1;55(23):5556–5560. [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996 Jun 15;10(12):1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- Konishi M., Kikuchi-Yanoshita R., Tanaka K., Muraoka M., Onda A., Okumura Y., Kishi N., Iwama T., Mori T., Koike M. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology. 1996 Aug;111(2):307–317. doi: 10.1053/gast.1996.v111.pm8690195. [DOI] [PubMed] [Google Scholar]
- Lane D. P. p53 and human cancers. Br Med Bull. 1994 Jul;50(3):582–599. doi: 10.1093/oxfordjournals.bmb.a072911. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K. W., Vogelstein B. Genetic instabilities in human cancers. Nature. 1998 Dec 17;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K. W., Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997 Apr 10;386(6625):623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- Leung S. Y., Yuen S. T., Chung L. P., Chu K. M., Chan A. S., Ho J. C. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999 Jan 1;59(1):159–164. [PubMed] [Google Scholar]
- Li G. M., Modrich P. Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1950–1954. doi: 10.1073/pnas.92.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom A., Tannergård P., Werelius B., Nordenskjöld M. Genetic mapping of a second locus predisposing to hereditary non-polyposis colon cancer. Nat Genet. 1993 Nov;5(3):279–282. doi: 10.1038/ng1193-279. [DOI] [PubMed] [Google Scholar]
- Liu B., Nicolaides N. C., Markowitz S., Willson J. K., Parsons R. E., Jen J., Papadopolous N., Peltomäki P., de la Chapelle A., Hamilton S. R. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995 Jan;9(1):48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R., Papadopoulos N., Nicolaides N. C., Lynch H. T., Watson P., Jass J. R., Dunlop M., Wyllie A., Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996 Feb;2(2):169–174. doi: 10.1038/nm0296-169. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lynch H. T., Krush A. J. Cancer family "G" revisited: 1895-1970. Cancer. 1971 Jun;27(6):1505–1511. doi: 10.1002/1097-0142(197106)27:6<1505::aid-cncr2820270635>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Lynch J. F. Genetics of colonic cancer. Digestion. 1998 Aug;59(5):481–492. doi: 10.1159/000007525. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Shaw M. W., Magnuson C. W., Larsen A. L., Krush A. J. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966 Feb;117(2):206–212. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer. 1996 Sep 15;78(6):1149–1167. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1149::AID-CNCR1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. An update of HNPCC (Lynch syndrome). Cancer Genet Cytogenet. 1997 Jan;93(1):84–99. doi: 10.1016/s0165-4608(96)00290-7. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Marsischky G. T., Filosi N., Kane M. F., Kolodner R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996 Feb 15;10(4):407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- Mecklin J. P., Järvinen H. J., Peltokallio P. Cancer family syndrome. Genetic analysis of 22 Finnish kindreds. Gastroenterology. 1986 Feb;90(2):328–333. [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Miyaki M., Konishi M., Tanaka K., Kikuchi-Yanoshita R., Muraoka M., Yasuno M., Igari T., Koike M., Chiba M., Mori T. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997 Nov;17(3):271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Okano M., Xie S., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998 Jul;19(3):219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Palombo F., Gallinari P., Iaccarino I., Lettieri T., Hughes M., D'Arrigo A., Truong O., Hsuan J. J., Jiricny J. GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science. 1995 Jun 30;268(5219):1912–1914. doi: 10.1126/science.7604265. [DOI] [PubMed] [Google Scholar]
- Palombo F., Iaccarino I., Nakajima E., Ikejima M., Shimada T., Jiricny J. hMutSbeta, a heterodimer of hMSH2 and hMSH3, binds to insertion/deletion loops in DNA. Curr Biol. 1996 Sep 1;6(9):1181–1184. doi: 10.1016/s0960-9822(02)70685-4. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Liu B., Parsons R., Lengauer C., Palombo F., D'Arrigo A., Markowitz S., Willson J. K., Kinzler K. W. Mutations of GTBP in genetically unstable cells. Science. 1995 Jun 30;268(5219):1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Vasen H. F. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997 Oct;113(4):1146–1158. doi: 10.1053/gast.1997.v113.pm9322509. [DOI] [PubMed] [Google Scholar]
- Percesepe A., Kristo P., Aaltonen L. A., Ponz de Leon M., de la Chapelle A., Peltomäki P. Mismatch repair genes and mononucleotide tracts as mutation targets in colorectal tumors with different degrees of microsatellite instability. Oncogene. 1998 Jul 16;17(2):157–163. doi: 10.1038/sj.onc.1201944. [DOI] [PubMed] [Google Scholar]
- Ponz de Leon M., Sassatelli R., Sacchetti C., Zanghieri G., Scalmati A., Roncucci L. Familial aggregation of tumors in the three-year experience of a population-based colorectal cancer registry. Cancer Res. 1989 Aug 1;49(15):4344–4348. [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991 Jan 11;251(4990):186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- Prolla T. A., Pang Q., Alani E., Kolodner R. D., Liskay R. M. MLH1, PMS1, and MSH2 interactions during the initiation of DNA mismatch repair in yeast. Science. 1994 Aug 19;265(5175):1091–1093. doi: 10.1126/science.8066446. [DOI] [PubMed] [Google Scholar]
- Rampino N., Yamamoto H., Ionov Y., Li Y., Sawai H., Reed J. C., Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997 Feb 14;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- Risinger J. I., Umar A., Boyd J., Berchuck A., Kunkel T. A., Barrett J. C. Mutation of MSH3 in endometrial cancer and evidence for its functional role in heteroduplex repair. Nat Genet. 1996 Sep;14(1):102–105. doi: 10.1038/ng0996-102. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bigas M. A., Boland C. R., Hamilton S. R., Henson D. E., Jass J. R., Khan P. M., Lynch H., Perucho M., Smyrk T., Sobin L. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997 Dec 3;89(23):1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- Rüschoff J., Dietmaier W., Lüttges J., Seitz G., Bocker T., Zirngibl H., Schlegel J., Schackert H. K., Jauch K. W., Hofstaedter F. Poorly differentiated colonic adenocarcinoma, medullary type: clinical, phenotypic, and molecular characteristics. Am J Pathol. 1997 May;150(5):1815–1825. [PMC free article] [PubMed] [Google Scholar]
- Sancar A. Excision repair invades the territory of mismatch repair. Nat Genet. 1999 Mar;21(3):247–249. doi: 10.1038/6753. [DOI] [PubMed] [Google Scholar]
- Sia E. A., Kokoska R. J., Dominska M., Greenwell P., Petes T. D. Microsatellite instability in yeast: dependence on repeat unit size and DNA mismatch repair genes. Mol Cell Biol. 1997 May;17(5):2851–2858. doi: 10.1128/mcb.17.5.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins S. B., Bocker T., Swisher E. M., Mutch D. G., Gersell D. J., Kovatich A. J., Palazzo J. P., Fishel R., Goodfellow P. J. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999 Apr;8(4):661–666. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- Souza R. F., Appel R., Yin J., Wang S., Smolinski K. N., Abraham J. M., Zou T. T., Shi Y. Q., Lei J., Cottrell J. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996 Nov;14(3):255–257. doi: 10.1038/ng1196-255. [DOI] [PubMed] [Google Scholar]
- Swale V. J., Quinn A. G., Wheeler J. M., Beck N. E., Dove-Edwin I., Thomas H. J., Bodmer W. F., Bataille V. A. Microsatellite instability in benign skin lesions in hereditary non-polyposis colorectal cancer syndrome. J Invest Dermatol. 1999 Dec;113(6):901–905. doi: 10.1046/j.1523-1747.1999.00788.x. [DOI] [PubMed] [Google Scholar]
- Syngal S., Fox E. A., Li C., Dovidio M., Eng C., Kolodner R. D., Garber J. E. Interpretation of genetic test results for hereditary nonpolyposis colorectal cancer: implications for clinical predisposition testing. JAMA. 1999 Jul 21;282(3):247–253. doi: 10.1001/jama.282.3.247. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., French A. J., Cunningham J. M., Tester D., Burgart L. J., Roche P. C., McDonnell S. K., Schaid D. J., Vockley C. W., Michels V. V. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998 Apr 15;58(8):1713–1718. [PubMed] [Google Scholar]
- Thibodeau S. N., French A. J., Roche P. C., Cunningham J. M., Tester D. J., Lindor N. M., Moslein G., Baker S. M., Liskay R. M., Burgart L. J. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996 Nov 1;56(21):4836–4840. [PubMed] [Google Scholar]
- Thorson A. G., Knezetic J. A., Lynch H. T. A century of progress in hereditary nonpolyposis colorectal cancer (Lynch syndrome). Dis Colon Rectum. 1999 Jan;42(1):1–9. doi: 10.1007/BF02235175. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Bodmer W. F. Failure of programmed cell death and differentiation as causes of tumors: some simple mathematical models. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11130–11134. doi: 10.1073/pnas.92.24.11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson I. P., Novelli M. R., Bodmer W. F. The mutation rate and cancer. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson I., Bodmer W. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat Med. 1999 Jan;5(1):11–12. doi: 10.1038/4687. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Watson P., Mecklin J. P., Lynch H. T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999 Jun;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Wijnen J. T., Menko F. H., Kleibeuker J. H., Taal B. G., Griffioen G., Nagengast F. M., Meijers-Heijboer E. H., Bertario L., Varesco L. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996 Apr;110(4):1020–1027. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- Veigl M. L., Kasturi L., Olechnowicz J., Ma A. H., Lutterbaugh J. D., Periyasamy S., Li G. M., Drummond J., Modrich P. L., Sedwick W. D. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P., Lynch H. T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993 Feb 1;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wheeler J. M., Beck N. E., Kim H. C., Tomlinson I. P., Mortensen N. J., Bodmer W. F. Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of hMLH1. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10296–10301. doi: 10.1073/pnas.96.18.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J., Leggett B., Gustafson C., Ward M., Searle J., Thomas L., Buttenshaw R., Chenevix-Trench G. Genomic instability occurs in colorectal carcinomas but not in adenomas. Hum Mutat. 1993;2(5):351–354. doi: 10.1002/humu.1380020505. [DOI] [PubMed] [Google Scholar]
- Zhang H., Richards B., Wilson T., Lloyd M., Cranston A., Thorburn A., Fishel R., Meuth M. Apoptosis induced by overexpression of hMSH2 or hMLH1. Cancer Res. 1999 Jul 1;59(13):3021–3027. [PubMed] [Google Scholar]