Abstract
BACKGROUND AND AIM—Intestinal epithelial cells (IEC) are thought to participate in the mucosal defence against bacteria and in the regulation of mucosal tissue homeostasis. Reactivity of IEC to bacterial signals may depend on interactions with immunocompetent cells. To address the question of whether non-pathogenic bacteria modify the immune response of the intestinal epithelium, we co-cultivated enterocyte-like CaCO-2 cells with human blood leucocytes in separate compartments of transwell cultures. METHODS—CaCO-2/PBMC co-cultures were stimulated with non-pathogenic bacteria and enteropathogenic Escherichia coli. Expression of tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-8, monocyte chemoattracting protein 1 (MCP-1), and IL-10 was studied by enzyme linked immunosorbent assays (cytokine secretion) and by semiquantitative reverse transcription-polymerase chain reaction. RESULTS—Challenge of CaCO-2 cells with non-pathogenic E coli and Lactobacillus sakei induced expression of IL-8, MCP-1, IL-1β, and TNF-α mRNA in the presence of underlying leucocytes. Leucocyte sensitised CaCO-2 cells produced TNF-α and IL-1β whereas IL-10 was exclusively secreted by human peripheral blood mononuclear cells. CaCO-2 cells alone remained hyporesponsive to the bacterial challenge. Lactobacillus johnsonii, an intestinal isolate, showed reduced potential to induce proinflammatory cytokines but increased transforming growth factor beta mRNA in leucocyte sensitised CaCO-2 cells. TNF-α was identified as one of the early mediators involved in cellular cross talk. In the presence of leucocytes, discriminative activation of CaCO-2 cells was observed between enteropathogenic E coli and non-pathogenic bacteria. CONCLUSION—The differential recognition of non-pathogenic bacteria by CaCO-2 cells required the presence of underlying leucocytes. These results strengthen the hypothesis that bacterial signalling at the mucosal surface is dependent on a network of cellular interactions. Keywords: CaCO-2 cells; leucocytes; enteropathogenic E coli; Lactobacilli; tumour necrosis factor; interleukin 1β; interleukin 10; chemokines
Full Text
The Full Text of this article is available as a PDF (229.9 KB).
Figure 1 .
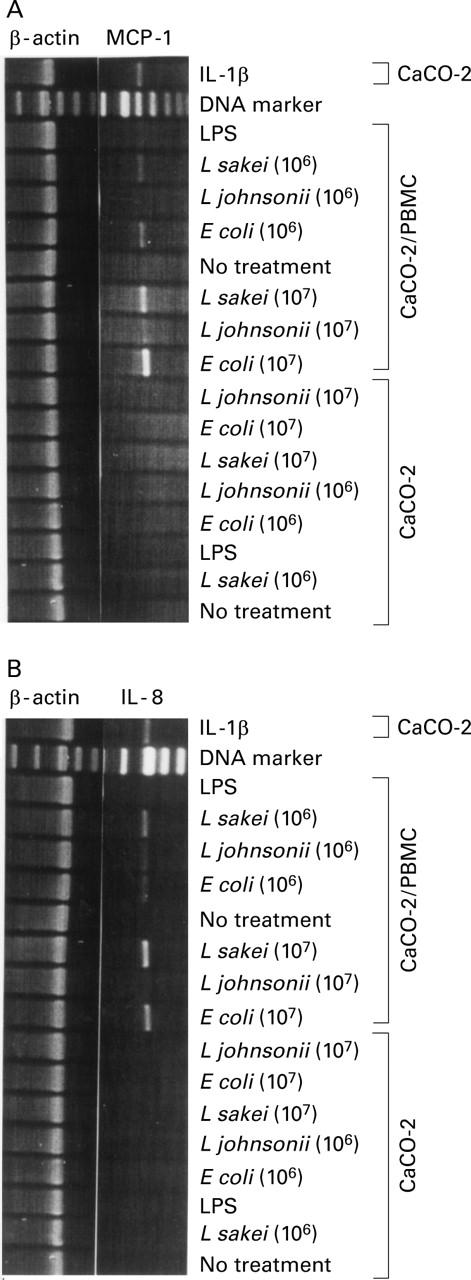
Differential chemokine expression in CaCO-2 cells. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to determine monocyte chemoattracting protein 1 (MCP-1) (A) and interleukin (IL)-8 (B) mRNA expression in CaCO-2 cells on stimulation of CaCO-2/leucocyte co-cultures or CaCO-2 cells alone with non-pathogenic E coli, L johnsonii, and L sakei (16 hours, 106 and 107 cfu/ml), respectively. Lipopolysaccharide (LPS, 1 µg/ml), IL-1β (10 ng/ml) and culture medium (no treatment) were used as controls. Results represent one of three independent experiments.
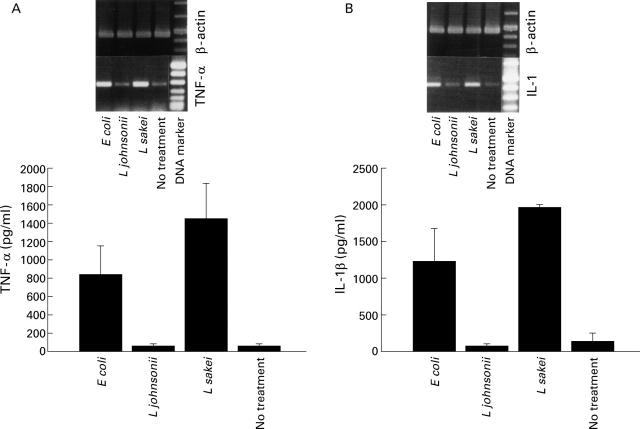
Figure 2 .
Bacteria induced tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1β response by CaCO-2/leucocyte co-cultures. Stimulation of CaCO-2/leucocyte co-cultures with non-pathogenic E coli, L johnsonii, and L sakei (16 hours, 107 cfu/ml). Secretion of TNF-α (A) and IL-1β (B) into the basolateral compartment was determined (bar charts). Culture medium (no treatment) was used as a control. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to determine expression of TNF-α (A) and IL-1β (B) mRNA in leucocyte sensitised CaCO-2 cells. Data are mean (SD) of triplicate values and represent one of three independent experiments.
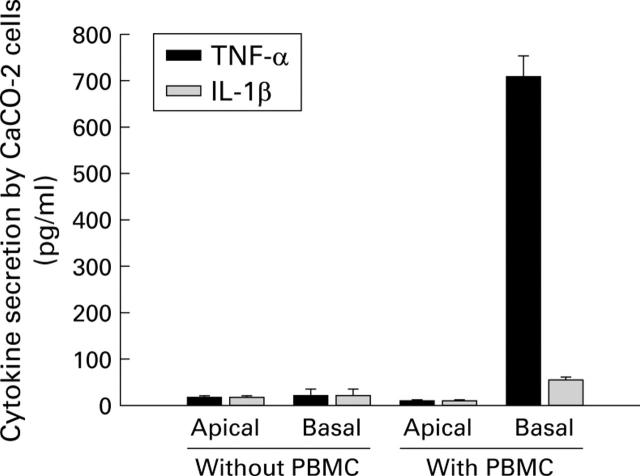
Figure 3 .
Polarised secretion of tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1β by leucocyte sensitised CaCO-2 cells. Stimulation of CaCO-2/leucocyte co-cultures or CaCO-2 cells alone with non-pathogenic E coli (107 cfu/ml). After 12 hours of incubation, CaCO-2 cells were transferred to fresh culture medium. After an additional 24 hours of incubation, secretion of TNF-α and IL-1β by leucocyte sensitised CaCO-2 cells was determined in the apical and basolateral compartments (transfer experiment). Controls (no treatment) did not induce cytokine secretion. Data are mean (SD) of triplicate values and represent one of three independent experiments.
Figure 4 .
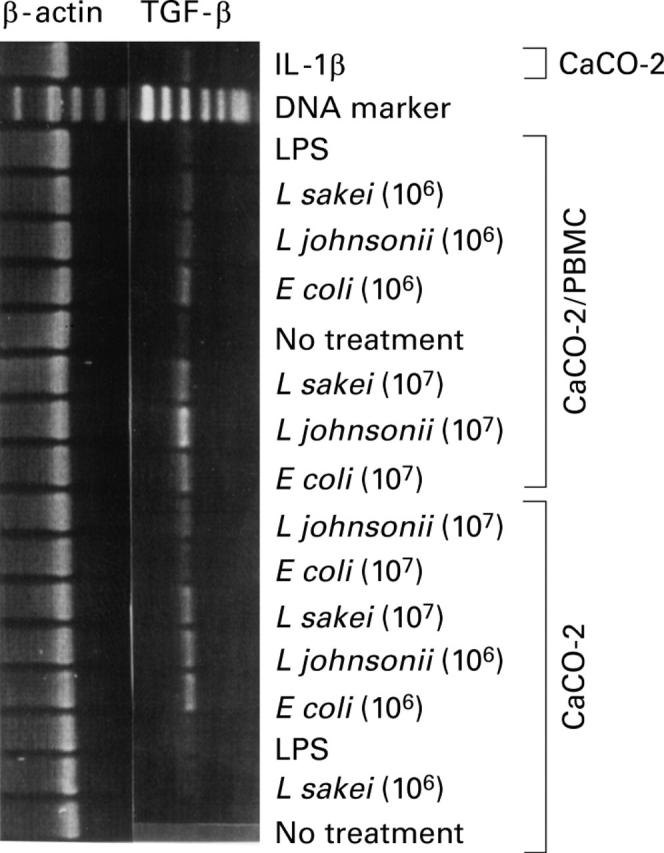
Differential expression of transforming growth factor beta (TGF-β) mRNA in CaCO-2 cells. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to determine TGF-β mRNA expression in CaCO-2 cells on stimulation of CaCO-2/leucocyte co-cultures or CaCO-2 cells alone with non-pathogenic E coli, L johnsonii, and L sakei (16 hours, 106 and 107 cfu/ml), respectively. Lipopolysaccharide (LPS, 1 µg/ml), interleukin (IL)-1β (10 ng/ml), and culture medium (no treatment) were used as controls. Results represent one of three independent experiments.
Figure 5 .
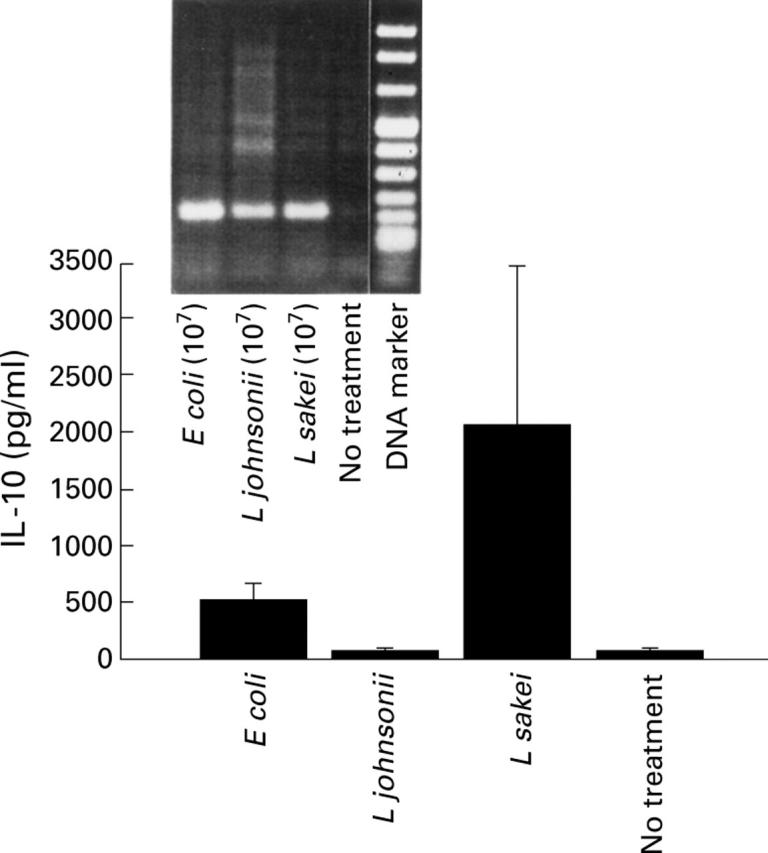
Induction of interleukin (IL)-10 response by CaCO-2/leucocyte co-cultures. Secretion of IL-10 was determined in the basolateral compartment on stimulation of CaCO-2/leucocyte co-cultures with non-pathogenic E coli, L johnsonii, and L sakei (16 hours, 107 cfu/ml), respectively (bar charts). Culture medium (no treatment) was used as a control. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to determine IL-10 mRNA expression in conditioned PBMC. Data are mean (SD) of triplicate values and represent one of three independent experiments.
Figure 6 .
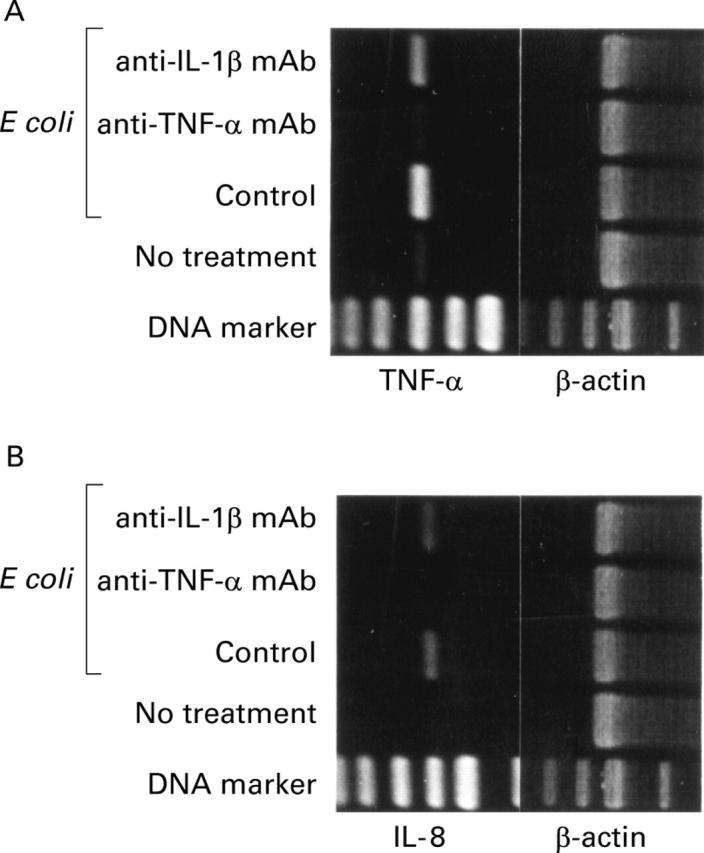
Neutralisation of tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1β in CaCO-2/leucocyte co-cultures. CaCO-2/ leucocyte co-cultures were stimulated with non-pathogenic E coli (16 hours, 107 cfu/ml) in the presence of neutralising anti-TNF-α monoclonal antibody (mAb) or IL-1β mAb (10 µg/ml). A positive control was performed in the absence of neutralising antibodies. Challenge of CaCO-2/leucocyte co-cultures with culture medium alone was used as a negative control. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to determine TNF-α (A) and IL-8 (B) mRNA expression in CaCO-2 cells. Results represent one of three independent experiments.
Figure 7 .
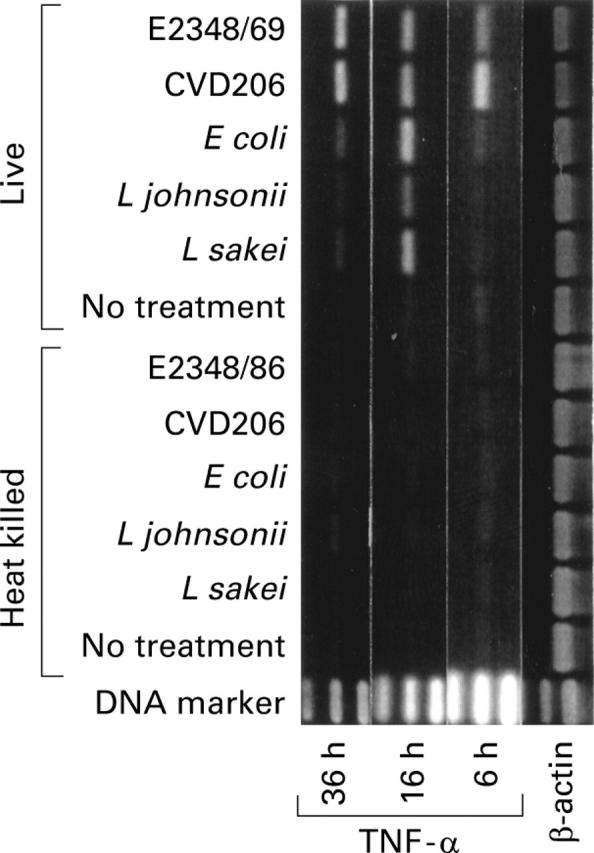
Differential induction of tumour necrosis factor alpha (TNF-α) mRNA by leucocyte sensitised CaCO-2 cells stimulated with enteropathogenic E coli and non-pathogenic bacteria. Stimulation of CaCO-2/leucocyte co-cultures with live or heat killed (95°C/30 minutes) EPEC strain E2348/69 and its eaeA deletion mutant CVD206, and non-pathogenic E coli, L johnsonii, and L sakei (107 cfu/ml) for four hours in the absence of gentamycin (150 µg/ml). Culture medium (no treatment) was used as a control. Expression of TNF-α mRNA in CaCO-2 cells was determined after 6, 16, and 36 hours of incubation by reverse transcription-polymerase chain reaction (RT-PCR) analysis. Results represent one of three independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh A., Fujiyama Y., Bamba T., Hosoda S. Differential cytokine regulation of complement C3, C4, and factor B synthesis in human intestinal epithelial cell line, Caco-2. J Immunol. 1993 Oct 15;151(8):4239–4247. [PubMed] [Google Scholar]
- Baert F. J., D'Haens G. R., Peeters M., Hiele M. I., Schaible T. F., Shealy D., Geboes K., Rutgeerts P. J. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999 Jan;116(1):22–28. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- Berg R. D., Savage D. C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975 Feb;11(2):320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J. R., Servin A. L. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994 Apr;35(4):483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg R. S., Terhorst C., Bleicher P., McDermott F. V., Allan C. H., Landau S. B., Trier J. S., Balk S. P. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J Immunol. 1991 Oct 15;147(8):2518–2524. [PubMed] [Google Scholar]
- Canil C., Rosenshine I., Ruschkowski S., Donnenberg M. S., Kaper J. B., Finlay B. B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993 Jul;61(7):2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A., Estes M. K., Crawford S. E., Ogra P. L., Ernst P. B., Garofalo R. P., Crowe S. E. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998 May;114(5):947–955. doi: 10.1016/s0016-5085(98)70314-2. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Chen W., Jin W., Cook M., Weiner H. L., Wahl S. M. Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J Immunol. 1998 Dec 1;161(11):6297–6304. [PubMed] [Google Scholar]
- Ciacci C., Mahida Y. R., Dignass A., Koizumi M., Podolsky D. K. Functional interleukin-2 receptors on intestinal epithelial cells. J Clin Invest. 1993 Jul;92(1):527–532. doi: 10.1172/JCI116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Growth of salmonellae in orally infected germfree mice. Infect Immun. 1978 Jul;21(1):41–47. doi: 10.1128/iai.21.1.41-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Duchmann R., Schmitt E., Knolle P., Meyer zum Büschenfelde K. H., Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996 Apr;26(4):934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Stenson W. F., Savidge T. C., Lowe D. C., Barrett K. E., Fierer J., Smith J. R., Kagnoff M. F. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin h synthase-2 expression and prostaglandin E2 and F2alpha production. J Clin Invest. 1997 Jul 15;100(2):296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Wang X., Barone F. C. Cytokines in brain ischemia--the role of TNF alpha. Cell Mol Neurobiol. 1998 Dec;18(6):695–701. doi: 10.1023/a:1020690004094. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Cytokines and intestinal inflammation. Transplant Proc. 1996 Oct;28(5):2442–2443. [PubMed] [Google Scholar]
- Graves D. T. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999 Mar;28(3):482–490. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeland L., Vaage J. T., Rolstad B., Midtvedt T., Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996 Dec;89(4):494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Poole S., Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996 Jun;60(2):316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberg R. M., Cho D. H., Youakim A., Bradley M. B., Lee J. S., Framson P. E., Nepom G. T. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998 Aug 15;102(4):792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberg R., Eghtesady P., Sydora B., Brorson K., Cheroutre H., Modlin R., Kronenberg M. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. T., Eckmann L., Savidge T. C., Kagnoff M. F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996 Jul 15;98(2):572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997 Jul 1;100(1):6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy R. Use of gnotobiotic pigs for research on enteritis associated with Escherichia coli. Proc R Soc Med. 1971 Apr;64(4):436–439. [PMC free article] [PubMed] [Google Scholar]
- Kernéis S., Bogdanova A., Kraehenbuhl J. P., Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997 Aug 15;277(5328):949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Kucharzik T., Lügering N., Pauels H. G., Domschke W., Stoll R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin Exp Immunol. 1998 Jan;111(1):152–157. doi: 10.1046/j.1365-2249.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent F., Eckmann L., Savidge T. C., Morgan G., Theodos C., Naciri M., Kagnoff M. F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997 Dec;65(12):5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Link H., Rochat F., Saudan K. Y., Schiffrin E. Immunomodulation of the gnotobiotic mouse through colonization with lactic acid bacteria. Adv Exp Med Biol. 1995;371A:465–467. doi: 10.1007/978-1-4615-1941-6_97. [DOI] [PubMed] [Google Scholar]
- Madsen K. L., Doyle J. S., Jewell L. D., Tavernini M. M., Fedorak R. N. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999 May;116(5):1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- McCormick B. A., Colgan S. P., Delp-Archer C., Miller S. I., Madara J. L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993 Nov;123(4):895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Parkos C. A., Colgan S. P., Carnes D. K., Madara J. L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998 Jan 1;160(1):455–466. [PubMed] [Google Scholar]
- McCormick B. A., Siber A. M., Maurelli A. T. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun. 1998 Sep;66(9):4237–4243. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. M., Croitoru K., Perdue M. H. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol. 1996 Feb;270(2 Pt 1):C418–C428. doi: 10.1152/ajpcell.1996.270.2.C418. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Weiner H. L. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991 Oct 1;174(4):791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Viney J. L. The anatomical basis of intestinal immunity. Immunol Rev. 1997 Apr;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Mühldorfer I., Blum G., Donohue-Rolfe A., Heier H., Olschläger T., Tschäpe H., Wallner U., Hacker J. Characterization of Escherichia coli strains isolated from environmental water habitats and from stool samples of healthy volunteers. Res Microbiol. 1996 Oct;147(8):625–635. doi: 10.1016/0923-2508(96)84019-8. [DOI] [PubMed] [Google Scholar]
- Panja A., Barone A., Mayer L. Stimulation of lamina propria lymphocytes by intestinal epithelial cells: evidence for recognition of nonclassical restriction elements. J Exp Med. 1994 Mar 1;179(3):943–950. doi: 10.1084/jem.179.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja A., Goldberg S., Eckmann L., Krishen P., Mayer L. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J Immunol. 1998 Oct 1;161(7):3675–3684. [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Guo J., Donati Y., Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998 Nov;28(11):3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Planchon S. M., Martins C. A., Guerrant R. L., Roche J. K. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994 Dec 15;153(12):5730–5739. [PubMed] [Google Scholar]
- Raitano A. B., Korc M. Growth inhibition of a human colorectal carcinoma cell line by interleukin 1 is associated with enhanced expression of gamma-interferon receptors. Cancer Res. 1993 Feb 1;53(3):636–640. [PubMed] [Google Scholar]
- Rasmussen S. J., Eckmann L., Quayle A. J., Shen L., Zhang Y. X., Anderson D. J., Fierer J., Stephens R. S., Kagnoff M. F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997 Jan 1;99(1):77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A., Cumano A., Vassalli P., Guy-Grand D., Kourilsky P. Oligoclonal repertoire of the CD8 alpha alpha and the CD8 alpha beta TCR-alpha/beta murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J Exp Med. 1994 Oct 1;180(4):1345–1358. doi: 10.1084/jem.180.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Podolsky D. K. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8353–8357. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997 Dec;92(12 Suppl):5S–11S. [PubMed] [Google Scholar]
- Savkovic S. D., Koutsouris A., Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997 Oct;273(4 Pt 1):C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Schreiber S. Interleukin-10 in the intestine. Gut. 1997 Aug;41(2):274–275. doi: 10.1136/gut.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerer-Maly C. C., Eckmann L., Kagnoff M. F., Falco M. T., Maly F. E. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994 Jan;81(1):85–91. [PMC free article] [PubMed] [Google Scholar]
- Schulte R., Wattiau P., Hartland E. L., Robins-Browne R. M., Cornelis G. R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996 Jun;64(6):2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Woolverton C. J., Holt L. C., Mitchell D., Sartor R. B. Identification and characterization of rat intestinal lamina propria cells: consequences of microbial colonization. Vet Immunol Immunopathol. 1992 Oct;34(1-2):127–138. doi: 10.1016/0165-2427(92)90156-k. [DOI] [PubMed] [Google Scholar]