Abstract
BACKGROUND—Collagenase-3 (matrix metalloproteinase-13, MMP-13) is a recently identified human MMP with broad substrate specificity which can be activated by membrane type 1 (MT1) matrix metalloproteinase in vitro. These may play a critical role in cancer aggressiveness. AIMS—To examine the clinical significance of collagenase-3 expression and the cooperative role of MT1-MMP in human oesophageal carcinomas. PATIENTS—Forty five individuals with oesophageal carcinoma who underwent surgery without preoperative treatment. METHODS—The tumour/normal (T/N) ratios of collagenase-3 and MT1-MMP mRNA expression in 45 human oesophageal carcinomas were determined by northern blot analysis. The production and localisation of collagenase-3 and MT1-MMP proteins were investigated by immunohistochemistry, western blot analysis, and zymography. RESULTS—The mean T/N ratio of collagenase-3 mRNA was 3.5 and that of MT1-MMP 2.1. There was a significant correlation between collagenase-3 and MT1-MMP mRNA expression (p<0.001). Twenty two cases with a collagenase-3 T/N ratio >3.5 showed a significantly higher frequency of vascular involvement and lymph node metastasis, and tended to be at a more advanced stage than 23 cases with a T/N ratio ⩽3.5 (p<0.05). Western blot analysis and zymography demonstrated production of collagenase-3 protein in tumour tissues but not in normal tissues. Immunohistochemical studies revealed that collagenase-3 was localised predominantly in tumour cells and MT1-MMP was detected in the same collagenase-3 positive cells; there was a significant association between collagenase-3 and MT1-MMP protein expression (p<0.05). With regard to prognosis, the survival time for subjects in the high collagenase-3 group (T/N ratio >3.5) was significantly worse (p<0.05) . CONCLUSIONS—These data suggest that production of collagenase-3 together with MT1-MMP is implicated in tumour aggressiveness and prognosis in human oesophageal carcinomas. Keywords: collagenase-3; MT1-MMP; oesophageal carcinoma; cancer aggressiveness; prognosis
Full Text
The Full Text of this article is available as a PDF (183.1 KB).
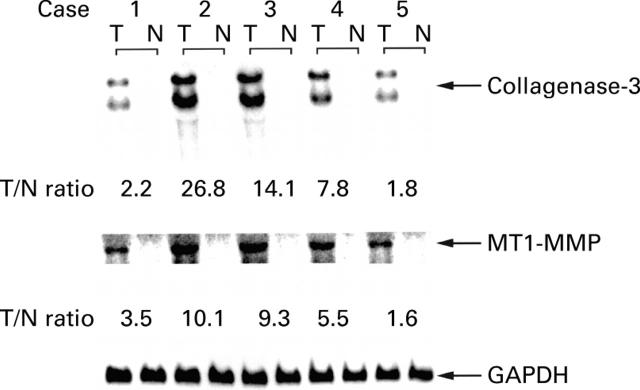
Figure 1 .
Total RNA isolated from the surgical specimens was analysed by northern blot hybridisation using a cDNA probe specific for human collagenase-3 (MMP-13), membrane type 1 matrix metalloproteinase (MT1-MMP), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Two intense bands (approximately 2.0 and 2.5 kb, respectively), present in the lanes probed for the RNA of collagenase-3 could be the result of utilisation of different polyadenylation sites. T/N ratio, tumour/normal ratio.
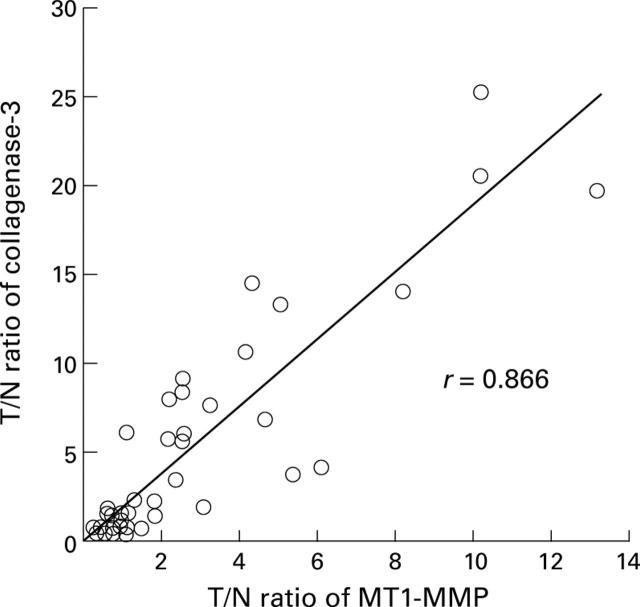
Figure 2 .
Plots of the tumour/normal ratio (T/N ratio) of collagenase-3 and the corresponding T/N ratio of membrane type 1 matrix metalloproteinase (MT1-MMP) for each case, showing a significant correlation (p<0.001).
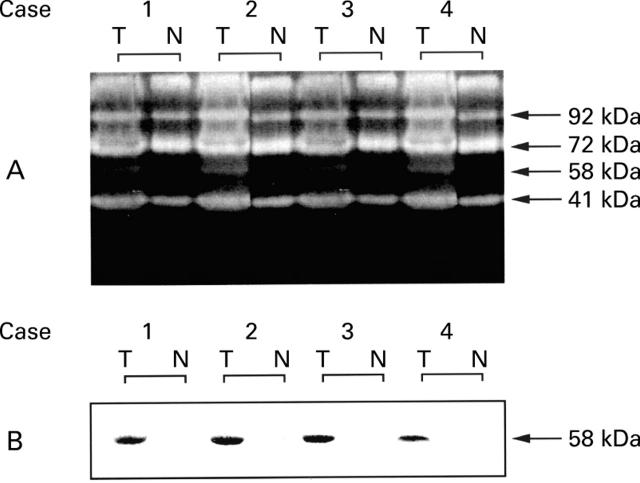
Figure 3 .
Protein isolated from the surgical specimens was analysed by gelatin zymography (A) and by western blot using specific collagenase-3 antibody (B), as described in the results section. T, tumour; N normal.
Figure 4 .
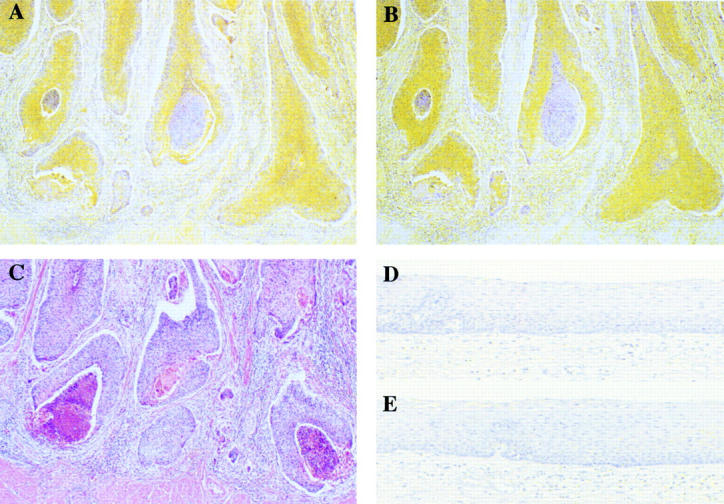
Immunohistochemical staining for collagenase-3 (A, D) and MT1-MMP (B, E) in human oesophageal carcinoma. (A) Collagenese-3 was strongly expressed in tumour cells, especially at the invasive front of the tumour cells. Also, weak expression was detected in stromal fibroblasts surrounding the tumour cells. (B) In a parallel section, MT1-MMP was detected mainly in tumour cells and weakly expressed in stromal fibroblasts surrounding the tumour cells. Also, MT1-MMP was detected in the same collagenase-3 positive tumour cells. (C) Haematoxylin-eosin staining in a parallel section. (D, E) Neither collagenase-3 nor MT1-MMP was expressed in normal tissues. Original magnification ×100.
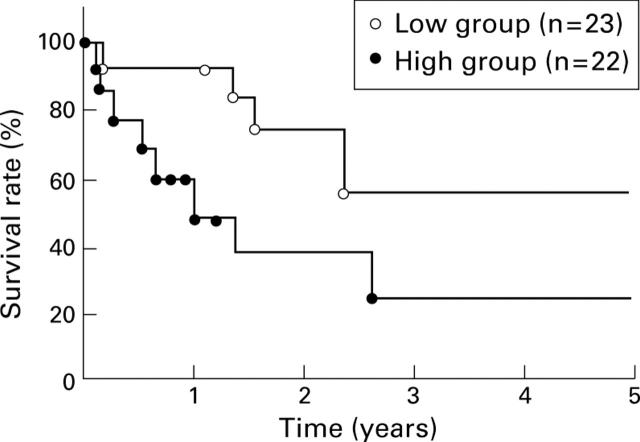
Figure 5 .
Survival curve for 45 patients with oesophageal cancer with respect to mRNA expression of collagenase-3. Patients with high collagenase-3 (MMP-13) expression (high group; tumour/normal (T/N) ratio >3.5) exhibited a significantly poorer prognosis than the low collagenase group (T/N⩽3.5) (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995 Oct;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Caenazzo C., Onisto M., Sartor L., Scalerta R., Giraldo A., Nitti D., Garbisa S. Augmented membrane type 1 matrix metalloproteinase (MT1-MMP):MMP-2 messenger RNA ratio in gastric carcinomas with poor prognosis. Clin Cancer Res. 1998 Sep;4(9):2179–2186. [PubMed] [Google Scholar]
- Cowell S., Knäuper V., Stewart M. L., D'Ortho M. P., Stanton H., Hembry R. M., López-Otín C., Reynolds J. J., Murphy G. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998 Apr 15;331(Pt 2):453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Waxman J., Wasan H., Abel P., Williams G., Krausz T., Neal D., Thomas D., Hanby A., Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993 Nov 15;53(22):5365–5369. [PubMed] [Google Scholar]
- Earlam R., Cunha-Melo J. R. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg. 1980 Jun;67(6):381–390. doi: 10.1002/bjs.1800670602. [DOI] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994 Jun 17;269(24):16766–16773. [PubMed] [Google Scholar]
- Haupt L. M., Thompson E. W., Griffiths L. R., Irving M. G. IS-RT-PCR assay detection of MT-MMP in a human breast cancer cell line. Biochem Mol Biol Int. 1996 Jun;39(3):553–561. doi: 10.1080/15216549600201611. [DOI] [PubMed] [Google Scholar]
- Isono K., Sato H., Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48(5):411–420. doi: 10.1159/000226971. [DOI] [PubMed] [Google Scholar]
- Johansson N., Airola K., Grénman R., Kariniemi A. L., Saarialho-Kere U., Kähäri V. M. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997 Aug;151(2):499–508. [PMC free article] [PubMed] [Google Scholar]
- Johansson N., Saarialho-Kere U., Airola K., Herva R., Nissinen L., Westermarck J., Vuorio E., Heino J., Kähäri V. M. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997 Mar;208(3):387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kanayama H., Yokota K., Kurokawa Y., Murakami Y., Nishitani M., Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998 Apr 1;82(7):1359–1366. [PubMed] [Google Scholar]
- Knäuper V., Will H., López-Otin C., Smith B., Atkinson S. J., Stanton H., Hembry R. M., Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996 Jul 19;271(29):17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- MacDougall J. R., Matrisian L. M. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995 Dec;14(4):351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Umenishi F., Funahashi K., Koshikawa N., Yasumitsu H., Umeda M. Activation of TIMP-2/progelatinase A complex by stromelysin. Biochem Biophys Res Commun. 1992 Jun 30;185(3):852–859. doi: 10.1016/0006-291x(92)91705-u. [DOI] [PubMed] [Google Scholar]
- Mori M., Barnard G. F., Mimori K., Ueo H., Akiyoshi T., Sugimachi K. Overexpression of matrix metalloproteinase-7 mRNA in human colon carcinomas. Cancer. 1995 Mar 15;75(6 Suppl):1516–1519. doi: 10.1002/1097-0142(19950315)75:6+<1516::aid-cncr2820751522>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Mori M., Barnard G. F., Staniunas R. J., Jessup J. M., Steele G. D., Jr, Chen L. B. Prothymosin-alpha mRNA expression correlates with that of c-myc in human colon cancer. Oncogene. 1993 Oct;8(10):2821–2826. [PubMed] [Google Scholar]
- Mori M., Mimori K., Shiraishi T., Fujie T., Baba K., Kusumoto H., Haraguchi M., Ueo H., Akiyoshi T. Analysis of MT1-MMP and MMP2 expression in human gastric cancers. Int J Cancer. 1997 Jun 20;74(3):316–321. doi: 10.1002/(sici)1097-0215(19970620)74:3<316::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Murray G. I., Duncan M. E., O'Neil P., McKay J. A., Melvin W. T., Fothergill J. E. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998 Jul;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Okada A., Bellocq J. P., Rouyer N., Chenard M. P., Rio M. C., Chambon P., Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani Y., Okazaki I., Arai M., Kameyama K., Wada N., Maruyama K., Yoshino K., Kitajima M., Hosoda Y., Tsuchiya M. Gene expression of interstitial collagenase (matrix metalloproteinase 1) in gastrointestinal tract cancers. J Gastroenterol. 1994 Aug;29(4):391–397. doi: 10.1007/BF02361233. [DOI] [PubMed] [Google Scholar]
- Sato H., Okada Y., Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in cell invasion. Thromb Haemost. 1997 Jul;78(1):497–500. [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sato Y., Mukai K., Furuya S., Kameya T., Hirohashi S. The AMeX method: a multipurpose tissue-processing and paraffin-embedding method. Extraction of protein and application to immunoblotting. Am J Pathol. 1992 Apr;140(4):775–779. [PMC free article] [PubMed] [Google Scholar]
- Shima I., Sasaguri Y., Kusukawa J., Yamana H., Fujita H., Kakegawa T., Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992 Dec 15;70(12):2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sugimachi K., Matsuoka H., Ohno S., Mori M., Kuwano H. Multivariate approach for assessing the prognosis of clinical oesophageal carcinoma. Br J Surg. 1988 Nov;75(11):1115–1118. doi: 10.1002/bjs.1800751122. [DOI] [PubMed] [Google Scholar]
- Tokuraku M., Sato H., Murakami S., Okada Y., Watanabe Y., Seiki M. Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer. 1995 Oct 20;64(5):355–359. doi: 10.1002/ijc.2910640513. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Airola K., Vaalamo M., Johansson N., Putnins E. E., Firth J. D., Salonen J., López-Otín C., Saarialho-Kere U., Kähäri V. M. Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol. 1998 Jun;152(6):1489–1499. [PMC free article] [PubMed] [Google Scholar]
- Uría J. A., Balbín M., López J. M., Alvarez J., Vizoso F., Takigawa M., López-Otín C. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am J Pathol. 1998 Jul;153(1):91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke D., Seyfert C., Hinzmann B., Gromnica-Ihle E. Cloning of collagenase 3 from the synovial membrane and its expression in rheumatoid arthritis and osteoarthritis. J Rheumatol. 1996 Apr;23(4):590–595. [PubMed] [Google Scholar]
- Yamamoto M., Mohanam S., Sawaya R., Fuller G. N., Seiki M., Sato H., Gokaslan Z. L., Liotta L. A., Nicolson G. L., Rao J. S. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. 1996 Jan 15;56(2):384–392. [PubMed] [Google Scholar]
- Yamashita K., Azumano I., Mai M., Okada Y. Expression and tissue localization of matrix metalloproteinase 7 (matrilysin) in human gastric carcinomas. Implications for vessel invasion and metastasis. Int J Cancer. 1998 Apr 17;79(2):187–194. doi: 10.1002/(sici)1097-0215(19980417)79:2<187::aid-ijc15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]