Abstract
BACKGROUND AND AIMS—Glucagon-like peptide-2 (GLP-2) is a recently identified potent intestinotrophic factor. We have evaluated the effect of GLP-2 treatment on intestinal epithelial barrier function in mice. METHODS—CD-1 mice were injected subcutaneously with GLP-2 or a protease resistant analogue, h[Gly2]GLP-2, twice daily for up to 10 days. Saline injected mice served as controls. Jejunal segments were mounted in Ussing chambers. Tissue conductance was measured and unidirectional fluxes were determined for (i) Na+ and the small inert probe Cr-EDTA (both transported via the paracellular pathway) and (ii) the macromolecule horseradish peroxidase (HRP, transported via the transcellular pathway). RESULTS—Mice treated with GLP-2 or h[Gly2]GLP-2 for 10 days demonstrated significantly reduced intestinal conductance and fluxes of Na+, Cr-EDTA, and HRP. Electron microscopy confirmed that GLP-2 reduced endocytic uptake of HRP into enterocytes. Functional changes (evident by four hours) preceded morphological changes (evident by 48 hours). CONCLUSIONS—GLP-2 enhances intestinal epithelial barrier function by affecting both paracellular and transcellular pathways and thus may be of therapeutic value in a number of gastrointestinal conditions. Keywords: intestinal permeability; macromolecular transport; growth factors
Full Text
The Full Text of this article is available as a PDF (249.0 KB).
Figure 1 .
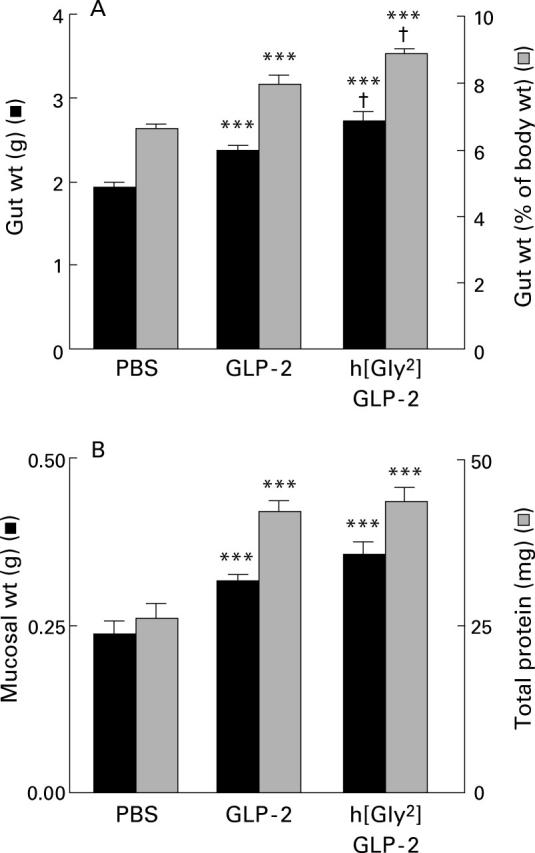
(A) Effect of GLP-2 or h[Gly2]GLP-2 treatment (5 µg injected on each of 10 days in mice) on wet weight of the small intestine or small intestinal weight as a percentage of body weight. (B) Effect of GLP-2 or h[Gly2]GLP-2 treatment for 10 days on weight of mucosal scrapings and protein content from a fixed length segment of murine intestine. Values are mean (SEM); n=7-8 mice/group. ***p<0.001 compared with control (phosphate buffered saline (PBS) treated mice); †p<0.05 compared with GLP-2 treated mice.
Figure 2 .
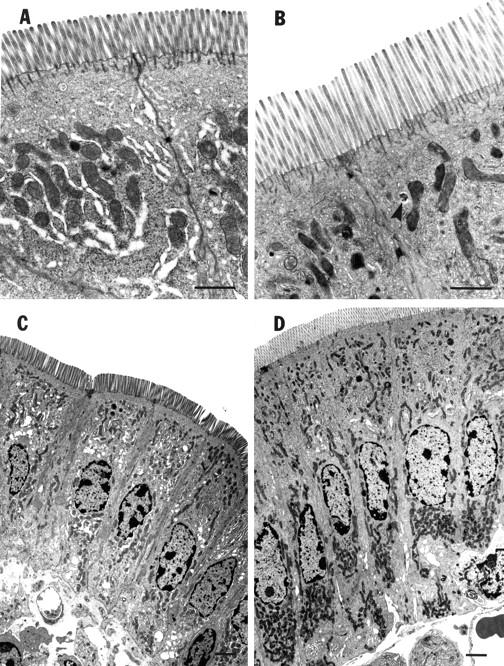
Representative photomicrographs of murine intestinal enterocytes. (A) and (C) are from phosphate buffered saline (PBS) treated mice (control); (B) and (D) are from mice after 10 days of treatment with h[Gly2]GLP-2 (5 µg). Note the longer microvilli in h[Gly2]GLP-2 treated mice (B) compared with controls (A) (bar=200 µm). The arrowhead in (B) indicates an endosome containing HRP. Note the increased cell length in h[Gly2]GLP-2 treated mice (D) compared with controls (C) (bar=100 µm).
Figure 3 .

Effect of GLP-2 or h[Gly2]GLP-2 treatment (5 µg injected on each of 10 days in mice) on (A) transepithelial ion conductance and (B) flux of 51Cr-EDTA across jejunal segments. Values are mean (SEM). (A) n=30-31 tissues from eight mice per group. (B) n=14-16 tissues from four mice per group.*p<0.05 compared with control (phosphate buffered saline (PBS) treated mice); †p<0.05 compared with GLP-2 treated mice.
Figure 4 .
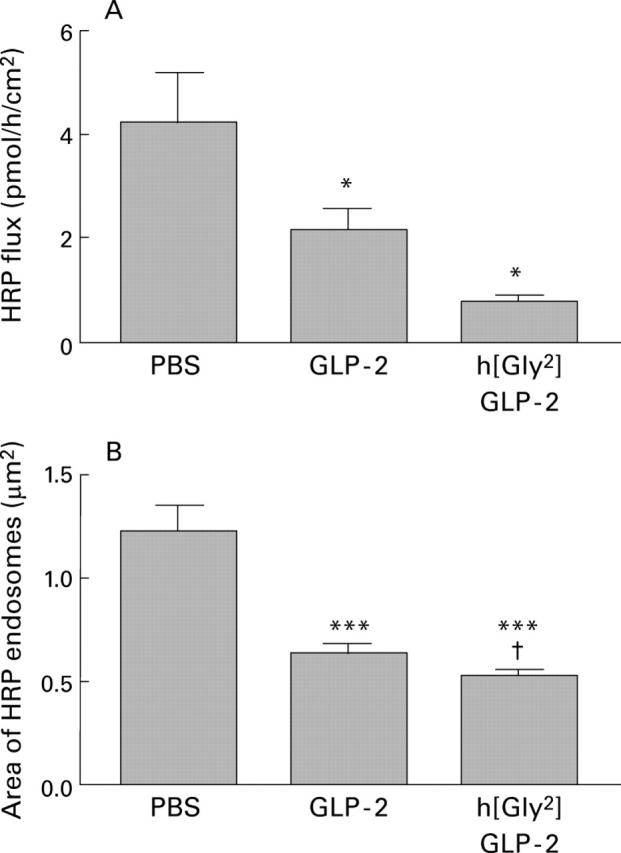
Effect of GLP-2 or h[Gly2]GLP-2 treatment (5 µg injected on each of 10 days in mice) on macromolecular permeability measurements following luminal addition of horseradish peroxidase (HRP 10−5 mol/l). (A) Flux of HRP across jejunal segments. Values are mean (SEM); n=12-15 tissues from 4-5 mice per group.*p<0.05 compared with control mice. (B) Area of HRP containing endosomes in enterocytes 60 minutes after HRP addition. Values are mean (SEM); n=12 photomicrographs per mouse, four mice per group (48 enterocytes). ***p<0.001 compared with control (phosphate buffered saline (PBS) treated mice); †p<0.05 compared with GLP-2 treated mice.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berin M. C., Kiliaan A. J., Yang P. C., Groot J. A., Taminiau J. A., Perdue M. H. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997 Sep;113(3):856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- Berin M. C., Yang P. C., Ciok L., Waserman S., Perdue M. H. Role for IL-4 in macromolecular transport across human intestinal epithelium. Am J Physiol. 1999 May;276(5 Pt 1):C1046–C1052. doi: 10.1152/ajpcell.1999.276.5.C1046. [DOI] [PubMed] [Google Scholar]
- Bijlsma P. B., Kiliaan A. J., Scholten G., Heyman M., Groot J. A., Taminiau J. A. Carbachol, but not forskolin, increases mucosal-to-serosal transport of intact protein in rat ileum in vitro. Am J Physiol. 1996 Jul;271(1 Pt 1):G147–G155. doi: 10.1152/ajpgi.1996.271.1.G147. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Peters T. J., Levi A. J. Intestinal permeability: clinical correlates. Dig Dis. 1986;4(2):83–92. doi: 10.1159/000171140. [DOI] [PubMed] [Google Scholar]
- Brubaker P. L., Izzo A., Hill M., Drucker D. J. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997 Jun;272(6 Pt 1):E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Foley-Nelson T., Thomas I., Balasubramaniam A. Prevention of parenteral nutrition-induced gut hypoplasia by coinfusion of glucagon-like peptide-2. Am J Physiol. 1997 Aug;273(2 Pt 1):G559–G563. doi: 10.1152/ajpgi.1997.273.2.G559. [DOI] [PubMed] [Google Scholar]
- Cheeseman C. I., Tsang R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol. 1996 Sep;271(3 Pt 1):G477–G482. doi: 10.1152/ajpgi.1996.271.3.G477. [DOI] [PubMed] [Google Scholar]
- Cheeseman C. I. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997 Dec;273(6 Pt 2):R1965–R1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Resnick M. B., Parkos C. A., Delp-Archer C., McGuirk D., Bacarra A. E., Weller P. F., Madara J. L. IL-4 directly modulates function of a model human intestinal epithelium. J Immunol. 1994 Sep 1;153(5):2122–2129. [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Drucker D. J., Erlich P., Asa S. L., Brubaker P. L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Shi Q., Crivici A., Sumner-Smith M., Tavares W., Hill M., DeForest L., Cooper S., Brubaker P. L. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997 Jul;15(7):673–677. doi: 10.1038/nbt0797-673. [DOI] [PubMed] [Google Scholar]
- Gasslander T., Permert J., Feng W., Adrian T. E., Larsson J. Trophic effects by epidermal growth factor on duodenal mucosa and exocrine pancreas in rats. Eur Surg Res. 1997;29(2):142–149. doi: 10.1159/000129518. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Bloom S. R., Polak J. M., Henry K., Dowling R. H. Endocrine tumour in kidney affecting small bowel structure, motility, and absorptive function. Gut. 1971 Oct;12(10):773–782. doi: 10.1136/gut.12.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go L. L., Healey P. J., Watkins S. C., Simmons R. L., Rowe M. I. The effect of endotoxin on intestinal mucosal permeability to bacteria in vitro. Arch Surg. 1995 Jan;130(1):53–58. doi: 10.1001/archsurg.1995.01430010055011. [DOI] [PubMed] [Google Scholar]
- Hadfield R. J., Sinclair D. G., Houldsworth P. E., Evans T. W. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995 Nov;152(5 Pt 1):1545–1548. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- Hirano M., Iwakiri R., Fujimoto K., Sakata H., Ohyama T., Sakai T., Joh T., Itoh M. Epidermal growth factor enhances repair of rat intestinal mucosa damaged by oral administration of methotrexate. J Gastroenterol. 1995 Apr;30(2):169–176. doi: 10.1007/BF02348661. [DOI] [PubMed] [Google Scholar]
- Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992 Sep;27(9):721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ma T. Y. Intestinal epithelial barrier dysfunction in Crohn's disease. Proc Soc Exp Biol Med. 1997 Apr;214(4):318–327. doi: 10.3181/00379727-214-44099. [DOI] [PubMed] [Google Scholar]
- Madara J. L. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989 Apr;83(4):1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. M., Croitoru K., Perdue M. H. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol. 1996 Feb;270(2 Pt 1):C418–C428. doi: 10.1152/ajpcell.1996.270.2.C418. [DOI] [PubMed] [Google Scholar]
- Meddings J. B. Review article: Intestinal permeability in Crohn's disease. Aliment Pharmacol Ther. 1997 Dec;11 (Suppl 3):47–56. doi: 10.1111/j.1365-2036.1997.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Peterson C. A., Ney D. M., Hinton P. S., Carey H. V. Beneficial effects of insulin-like growth factor I on epithelial structure and function in parenterally fed rat jejunum. Gastroenterology. 1996 Dec;111(6):1501–1508. doi: 10.1016/s0016-5085(96)70011-2. [DOI] [PubMed] [Google Scholar]
- Philpott D. J., McKay D. M., Sherman P. M., Perdue M. H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996 Apr;270(4 Pt 1):G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- Planchon S. M., Martins C. A., Guerrant R. L., Roche J. K. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994 Dec 15;153(12):5730–5739. [PubMed] [Google Scholar]
- Rodriguez P., Heyman M., Candalh C., Blaton M. A., Bouchaud C. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl.19A cell monolayers. Cytokine. 1995 Jul;7(5):441–448. doi: 10.1006/cyto.1995.0060. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995 Sep;24(3):475–507. [PubMed] [Google Scholar]
- Saunders P. R., Kosecka U., McKay D. M., Perdue M. H. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994 Nov;267(5 Pt 1):G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- Scott R. B., Kirk D., MacNaughton W. K., Meddings J. B. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998 Nov;275(5 Pt 1):G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Hill M., Asa S. L., Brubaker P. L., Drucker D. J. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997 Jul;273(1 Pt 1):E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Hill M., Drucker D. J. Biological determinants of intestinotrophic properties of GLP-2 in vivo. Am J Physiol. 1997 Mar;272(3 Pt 1):G662–G668. doi: 10.1152/ajpgi.1997.272.3.G662. [DOI] [PubMed] [Google Scholar]
- Wallace J. L. NSAID gastroenteropathy: past, present and future. Can J Gastroenterol. 1996 Nov-Dec;10(7):451–459. doi: 10.1155/1996/850710. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. J., Watts M. T., Bhatt B. D., Ho H. Intestinal permeability to [51Cr]EDTA in infectious diarrhea. Dig Dis Sci. 1993 Sep;38(9):1651–1657. doi: 10.1007/BF01303174. [DOI] [PubMed] [Google Scholar]


