Abstract
BACKGROUND/AIMS—In 1998 the PPP2R1B gene encoding the A subunit of the serine/threonine protein phosphatase was identified as a putative tumour suppressor gene in lung and colon cancer in the chromosome region 11q22-24. The aim of the present study was to determine the type of alterations in primary rectal cancers as well as colon cancers and the correlation between these alterations and clinicopathological data. METHODS—Mutation analyses of the PPP2R1B gene sequence encoding the binding sites of the catalytic C subunit (Huntington elongation A subunit TOR (HEAT) repeats 11-15) and partial binding sites of the regulatory B subunit were carried out on cDNA samples from 30 primary colorectal cancer specimens and corresponding normal tissues using a combination of the polymerase chain reaction and subsequent direct DNA sequencing. RESULTS—Five missense mutations producing amino acid substitutions were detected in the four colon cancer cases (13.3%; four of 30 colorectal cancers): 15glycine (GGT) to alanine (GCT) and 499leucine (TTA) to isoleucine (ATA) in the same case, and 498valine (GTG) to glutamic acid (GAG), 500valine (GTA) to glycine (GGA), and 365serine (TCT) to proline (CCT). Of these five mutations, three (60%) were located in HEAT repeat 13 and four (80%) showed T to other nucleotide substitutions. In addition, a normal polymorphism, 478leucine, was found. No correlation was found between these mutations and clinicopathological data. CONCLUSION—Our results suggest that the PPP2R1B gene is one of the true targets at 11q23, and its inactivation is involved in the development of all types of colorectal cancers. Keywords: PPP2R1B gene; colorectal cancer; tumour suppressor gene; protein phosphatase
Full Text
The Full Text of this article is available as a PDF (227.6 KB).
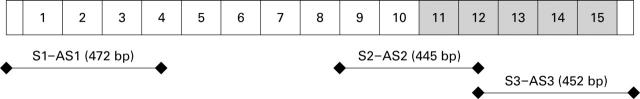
Figure 1 .
Schematic view of PPP2R1B cDNAs. Three sets of polymerase chain reaction primers were used to amplify a portion of the gene. Numbers indicate the internal repeats (HEAT repeats) and shaded numbers indicate the sequence of binding sites for the PP2Ac subunit on the PR65/A subunit.
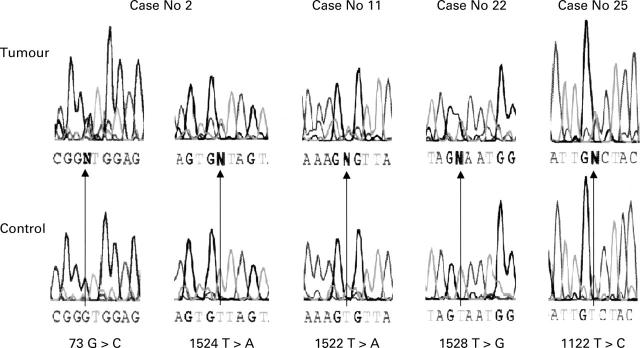
Figure 2 .
DNA sequence electropherograms showing the PPP2R1B gene mutations identified in patients with colorectal cancer. Individual electropherograms for the tumour are shown in the upper panels and the corresponding normal tissues (controls) are shown in the lower panels. The relevant nucleotide is indicated by an arrow.
Figure 3 .
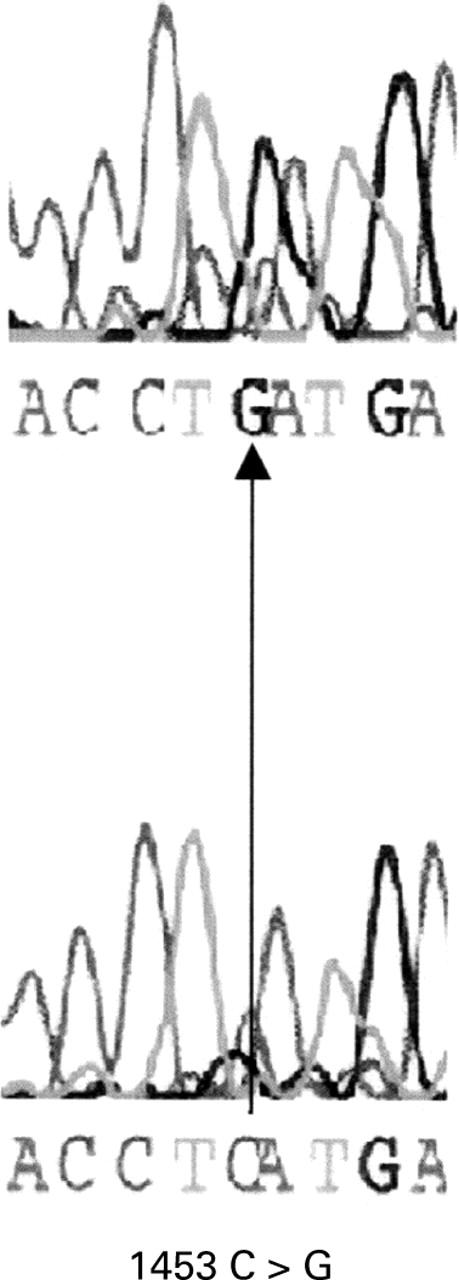
DNA sequence showing a single base substitution without amino acid substitution of the PPP2R1B gene. The consensus sequence is shown in the upper panel and an identified substitution is shown in the lower panel.
Figure 4 .
Multiple sequence alignment of HEAT motif sequences and location of the mutations and polymorphism. Boxed amino acids represent highly conserved residues and shaded amino acids indicate hydrophobic residues. Amino acids underlined are mutated except for L ( 478Leu ) in HEAT repeat 12.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Béroud C., Soussi T. p53 gene mutation: software and database. Nucleic Acids Res. 1998 Jan 1;26(1):200–204. doi: 10.1093/nar/26.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. R., Hanlon N., Turowski P., Hemmings B. A., Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999 Jan 8;96(1):99–110. doi: 10.1016/s0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Gustafson C. E., Young J., Leggett B., Searle J., Chenevix-Trench G. Loss of heterozygosity on the long arm of chromosome 11 in colorectal tumours. Br J Cancer. 1994 Sep;70(3):395–397. doi: 10.1038/bjc.1994.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Solomon M. J., Mumby M. C., Kirschner M. W. INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell. 1991 Jan 25;64(2):415–423. doi: 10.1016/0092-8674(91)90649-j. [DOI] [PubMed] [Google Scholar]
- Li H., Zhao L. L., Funder J. W., Liu J. P. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem. 1997 Jul 4;272(27):16729–16732. doi: 10.1074/jbc.272.27.16729. [DOI] [PubMed] [Google Scholar]
- Monaco C., Negrini M., Sozzi G., Veronese M. L., Vorechovsky I., Godwin A. K., Croce C. M. Molecular cloning and characterization of LOH11CR2A, a new gene within a refined minimal region of LOH at 11q23. Genomics. 1997 Dec 1;46(2):217–222. doi: 10.1006/geno.1997.5036. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993 Oct;73(4):673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Negrini M., Rasio D., Hampton G. M., Sabbioni S., Rattan S., Carter S. L., Rosenberg A. L., Schwartz G. F., Shiloh Y., Cavenee W. K. Definition and refinement of chromosome 11 regions of loss of heterozygosity in breast cancer: identification of a new region at 11q23.3. Cancer Res. 1995 Jul 15;55(14):3003–3007. [PubMed] [Google Scholar]
- Rasio D., Negrini M., Croce C. M. Genomic organization of the ATM locus involved in ataxia-telangiectasia. Cancer Res. 1995 Dec 15;55(24):6053–6057. [PubMed] [Google Scholar]
- Rasio D., Negrini M., Manenti G., Dragani T. A., Croce C. M. Loss of heterozygosity at chromosome 11q in lung adenocarcinoma: identification of three independent regions. Cancer Res. 1995 Sep 15;55(18):3988–3991. [PubMed] [Google Scholar]
- Ruediger R., Hentz M., Fait J., Mumby M., Walter G. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J Virol. 1994 Jan;68(1):123–129. doi: 10.1128/jvi.68.1.123-129.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger R., Roeckel D., Fait J., Bergqvist A., Magnusson G., Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992 Nov;12(11):4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Wong C., Thoma R. S., Richman R., Wu Z., Piwnica-Worms H., Elledge S. J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997 Sep 5;277(5331):1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Ziv Y., Bar-Shira A., Gilad S., Tagle D. A., Smith S., Uziel T., Sfez S., Nahmias J., Sartiel A. A human gene (DDX10) encoding a putative DEAD-box RNA helicase at 11q22-q23. Genomics. 1996 Apr 15;33(2):199–206. doi: 10.1006/geno.1996.0184. [DOI] [PubMed] [Google Scholar]
- Schönthal A. H. Role of PP2A in intracellular signal transduction pathways. Front Biosci. 1998 Dec 15;3:D1262–D1273. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Bodmer W. F. Chromosome 11q in sporadic colorectal carcinoma: patterns of allele loss and their significance for tumorigenesis. J Clin Pathol. 1996 May;49(5):386–390. doi: 10.1136/jcp.49.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ferre F., Espiritu O., Carbone-Wiley A. Molecular cloning and sequence of cDNA encoding polyoma medium tumor antigen-associated 61-kDa protein. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8669–8672. doi: 10.1073/pnas.86.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Esplin E. D., Li J. L., Huang L., Gazdar A., Minna J., Evans G. A. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998 Oct 9;282(5387):284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- Wang S. S., Virmani A., Gazdar A. F., Minna J. D., Evans G. A. Refined mapping of two regions of loss of heterozygosity on chromosome band 11q23 in lung cancer. Genes Chromosomes Cancer. 1999 Jun;25(2):154–159. [PubMed] [Google Scholar]
- Ziemin-van der Poel S., McCabe N. R., Gill H. J., Espinosa R., 3rd, Patel Y., Harden A., Rubinelli P., Smith S. D., LeBeau M. M., Rowley J. D. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]