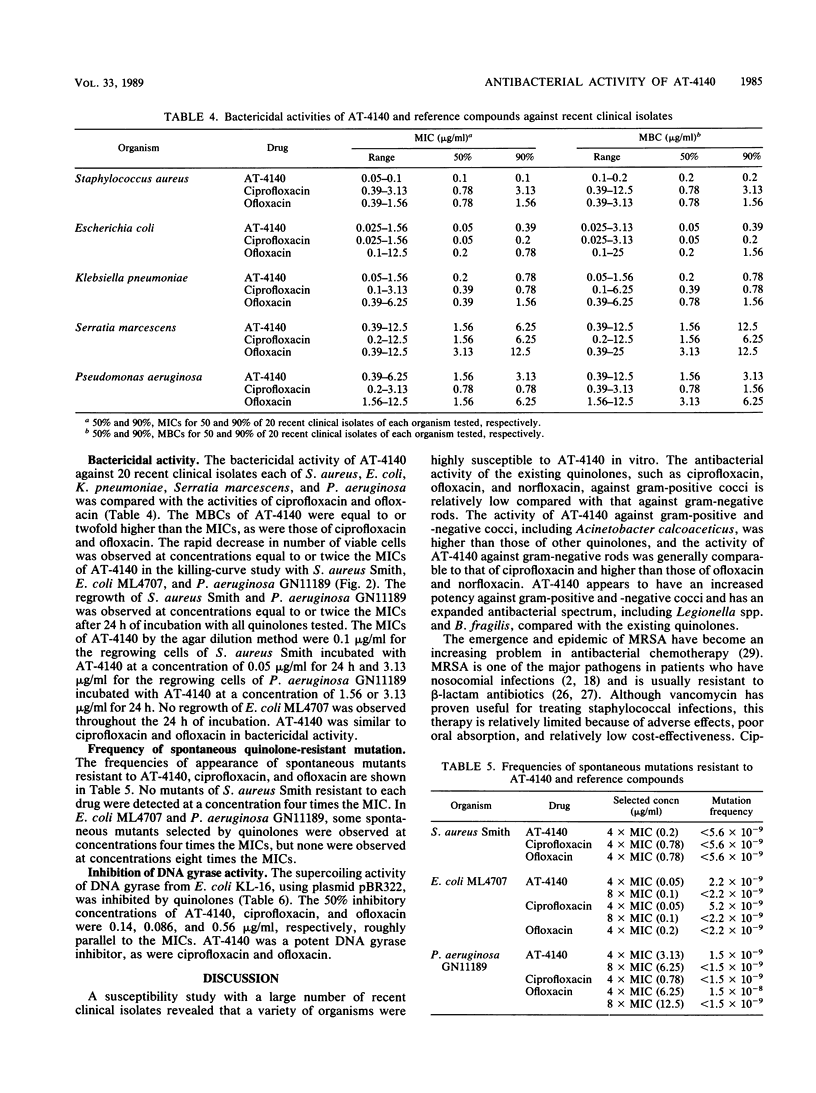
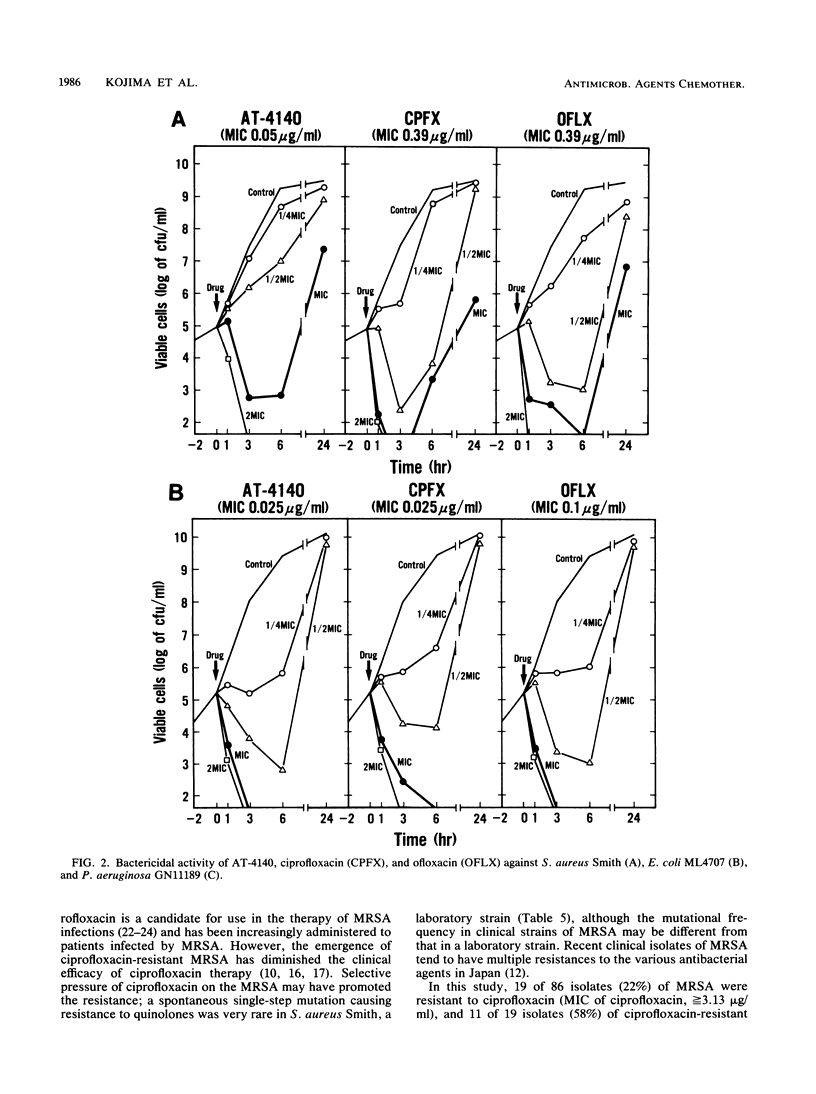
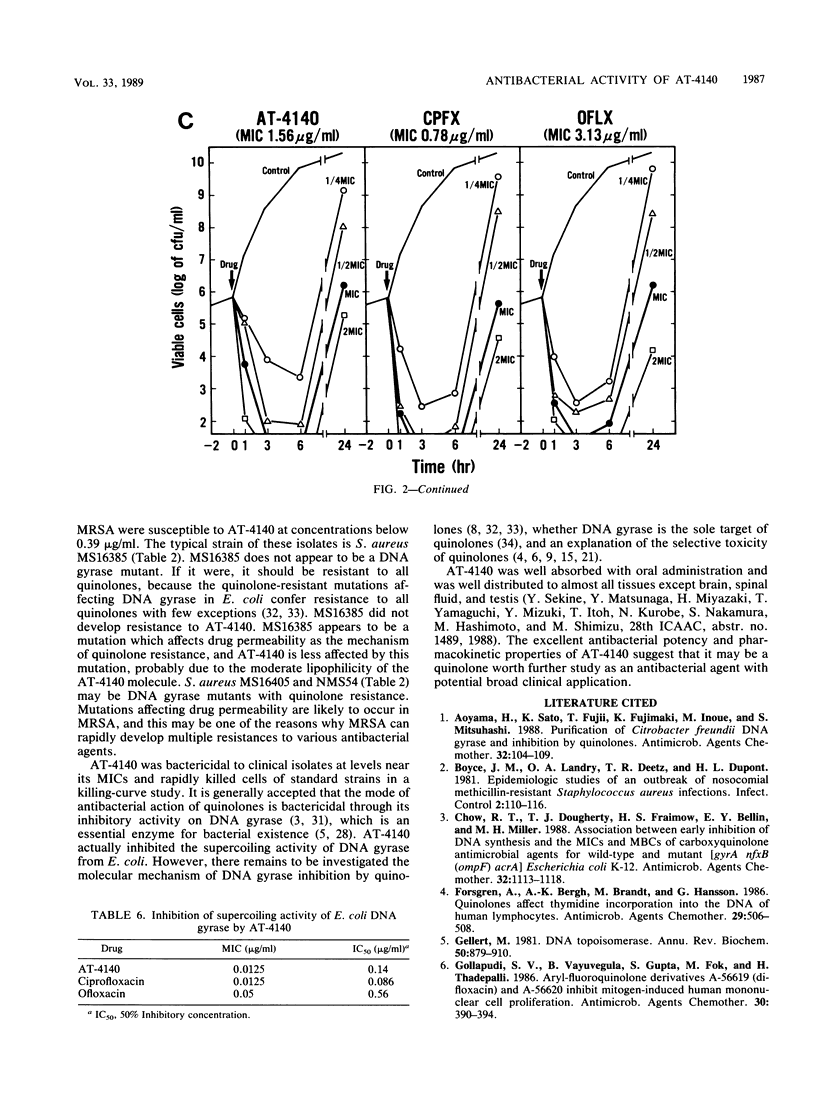
Abstract
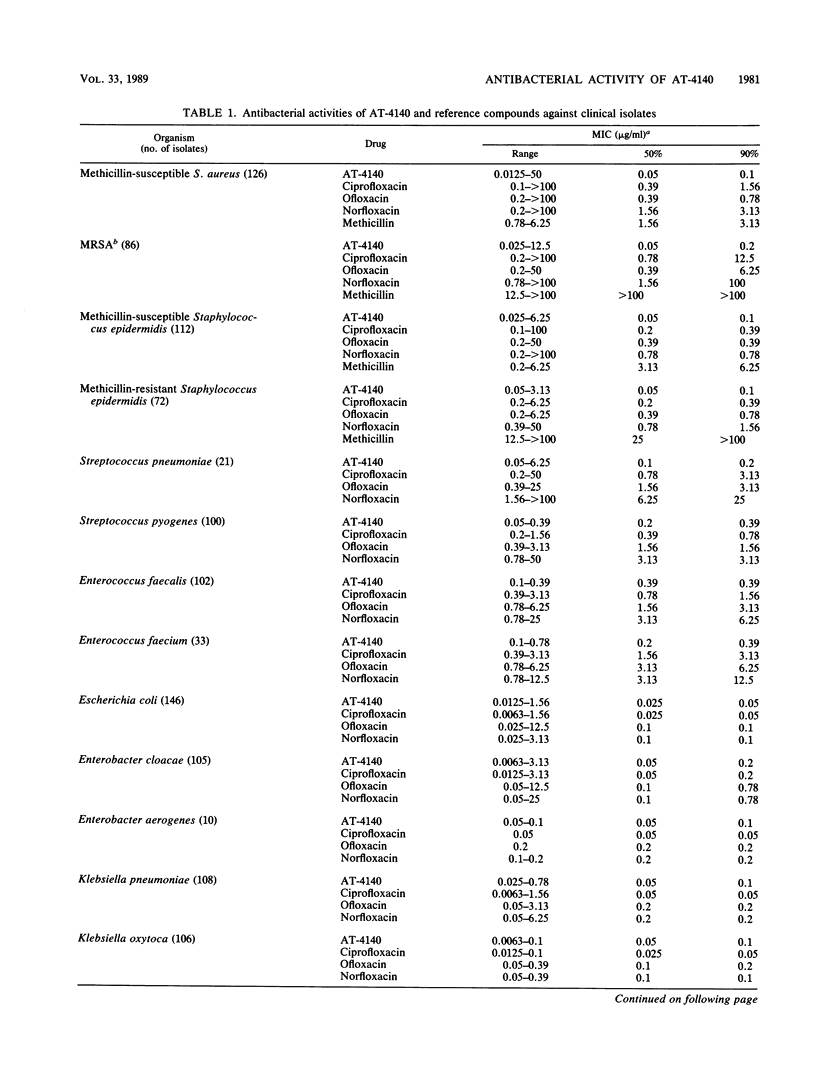
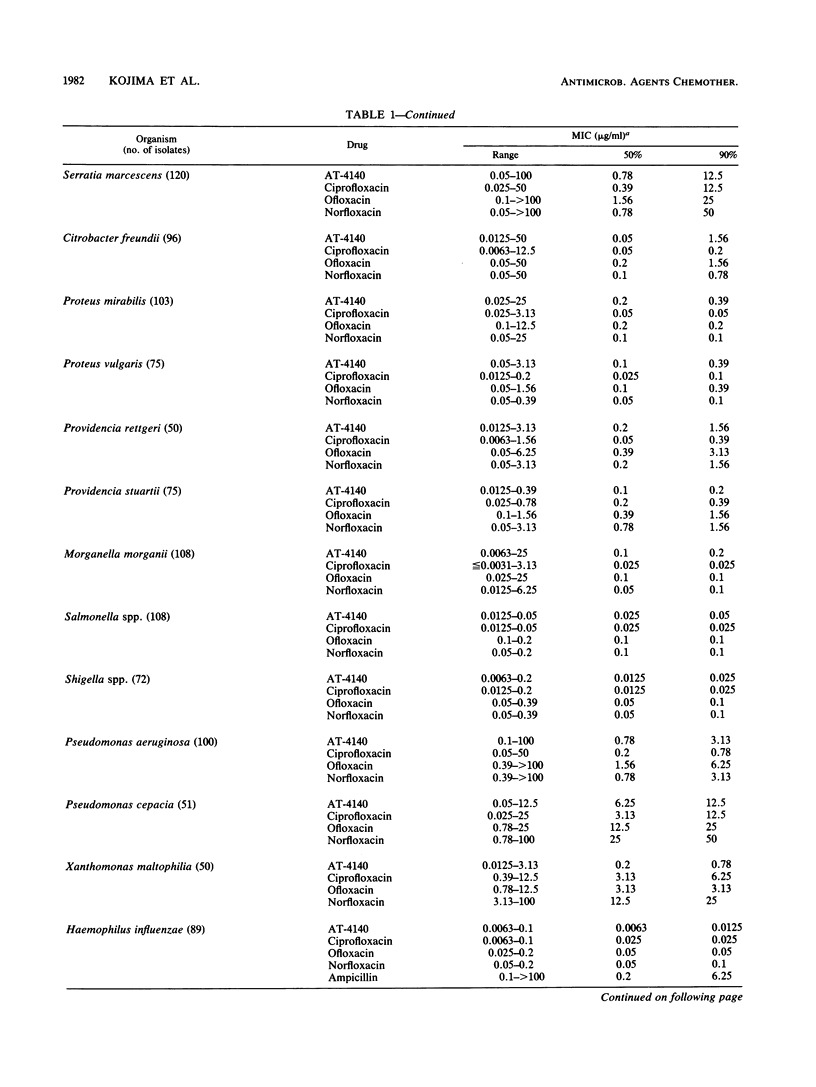
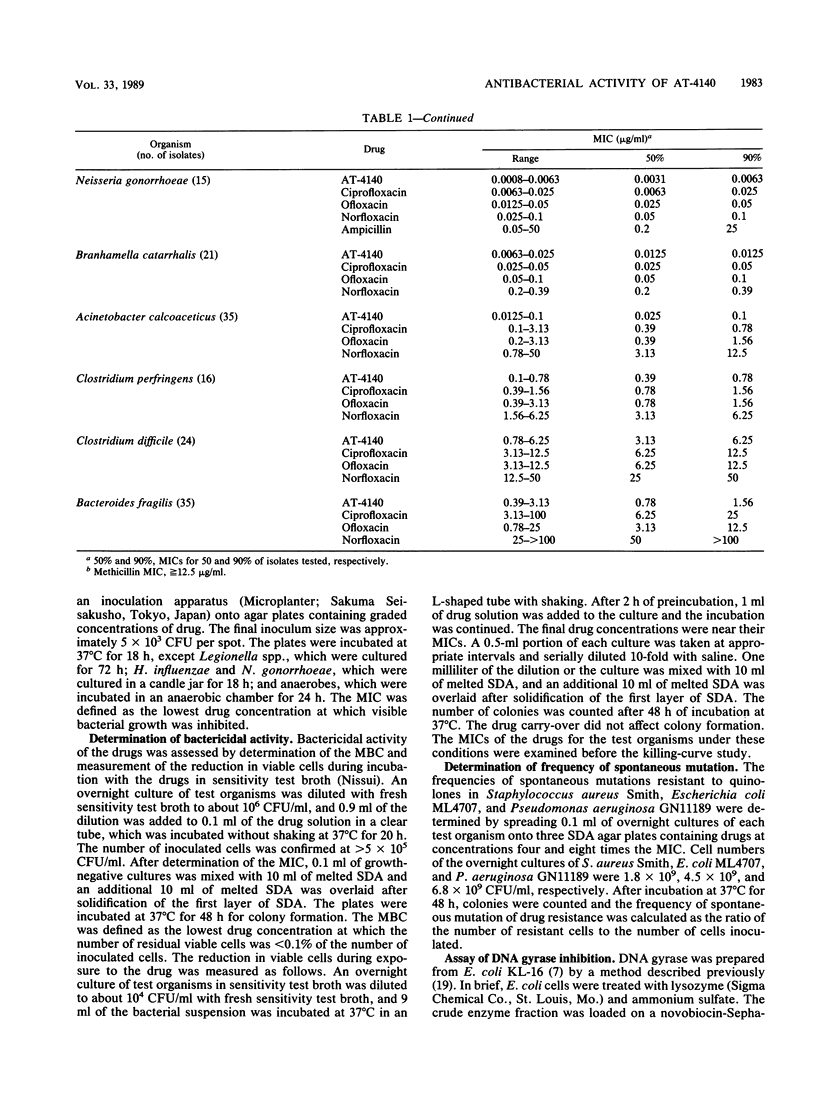
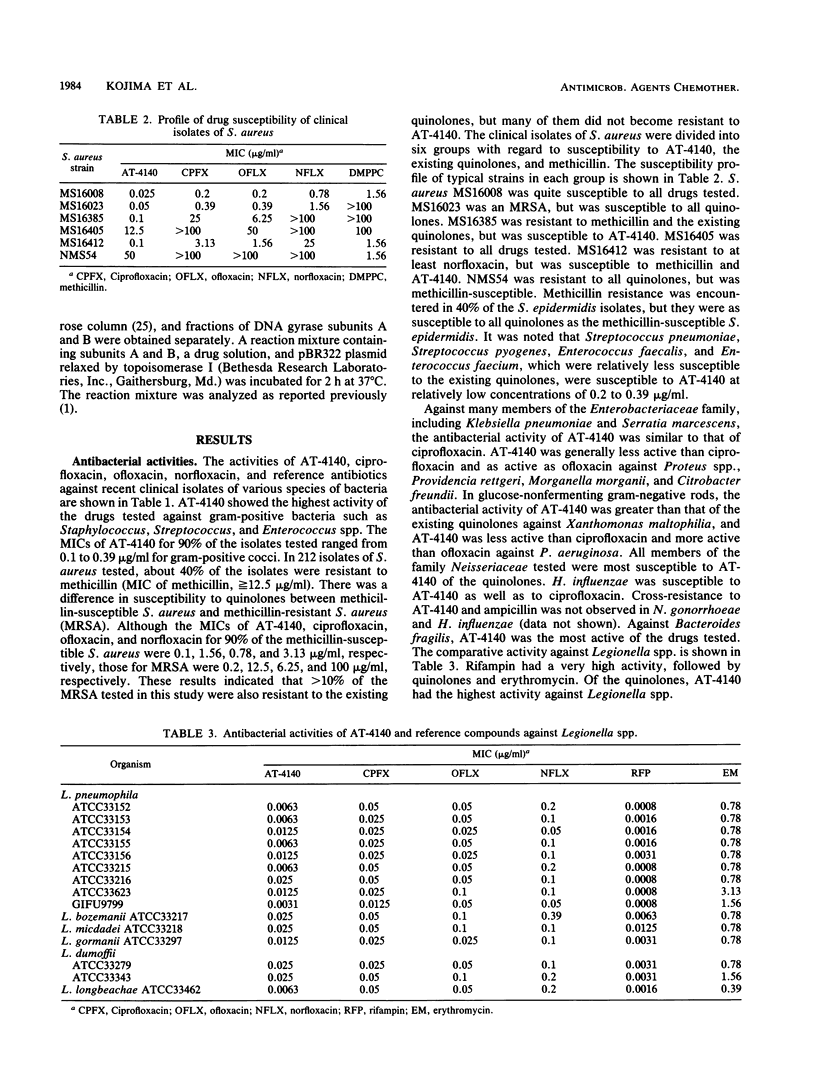
The activity of AT-4140, a new fluoroquinolone, was evaluated against a wide range of clinical bacterial isolates and compared with those of existing analogs. AT-4140 had a broad spectrum and a potent activity against gram-positive and -negative bacteria, including Legionella spp. and Bacteroides fragilis. The activity of AT-4140 against gram-positive and -negative cocci, including Acinetobacter calcoaceticus, was higher than those of ciprofloxacin, ofloxacin, and norfloxacin. Its activity against gram-negative rods was generally comparable to that of ciprofloxacin. Some isolates of methicillin-resistant Staphylococcus aureus (MIC of methicillin, greater than or equal to 12.5 micrograms/ml) were resistant to existing quinolones, but many of them were still susceptible to AT-4140 at concentrations below 0.39 micrograms/ml. The MICs of AT-4140, ciprofloxacin, ofloxacin, and norfloxacin for 90% of clinical isolates of methicillin-resistant S. aureus were 0.2, 12.5, 6.25, and 100 micrograms/ml, respectively. AT-4140 was bactericidal for each of 20 clinical isolates of Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, and Pseudomonas aeruginosa at concentrations near the MICs. AT-4140 inhibited the supercoiling activity of DNA gyrase from E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Sato K., Fujii T., Fujimaki K., Inoue M., Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition by quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., Landry M., Deetz T. R., DuPont H. L. Epidemiologic studies of an outbreak of nosocomial methicillin-resistant Staphylococcus aureus infections. Infect Control. 1981 Mar-Apr;2(2):110–116. doi: 10.1017/s0195941700053881. [DOI] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Bergh A. K., Brandt M., Hansson G. Quinolones affect thymidine incorporation into the DNA of human lymphocytes. Antimicrob Agents Chemother. 1986 Mar;29(3):506–508. doi: 10.1128/aac.29.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gollapudi S. V., Vayuvegula B., Gupta S., Fok M., Thadepalli H. Aryl-fluoroquinolone derivatives A-56619 (difloxacin) and A-56620 inhibit mitogen-induced human mononuclear cell proliferation. Antimicrob Agents Chemother. 1986 Sep;30(3):390–394. doi: 10.1128/aac.30.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouno K., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activity of AT-2266. Antimicrob Agents Chemother. 1983 Jul;24(1):78–84. doi: 10.1128/aac.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESHER G. Y., FROELICH E. J., GRUETT M. D., BAILEY J. H., BRUNDAGE R. P. 1,8-NAPHTHYRIDINE DERIVATIVES. A NEW CLASS OF CHEMOTHERAPEUTIC AGENTS. J Med Pharm Chem. 1962 Sep;91:1063–1065. doi: 10.1021/jm01240a021. [DOI] [PubMed] [Google Scholar]
- Mitelman F., Kolnig A. M., Strömbeck B., Norrby R., Kromann-Andersen B., Sommer P., Wadstein J. No cytogenetic effects of quinolone treatment in humans. Antimicrob Agents Chemother. 1988 Jun;32(6):936–937. doi: 10.1128/aac.32.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Ruane P. J., Johnston L., Wong P., Wheelock J. P., MacDonald K., Reinhardt J. F., Johnson C. C., Statner B., Blomquist I. Ciprofloxacin for eradication of methicillin-resistant Staphylococcus aureus colonization. Am J Med. 1987 Apr 27;82(4A):215–219. [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravolatz L. D., Pohlod D. J., Arking L. M. Community-acquired methicillin-resistant Staphylococcus aureus infections: a new source for nosocomial outbreaks. Ann Intern Med. 1982 Sep;97(3):325–329. doi: 10.7326/0003-4819-97-3-325. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Rahal J. J., Jr Bactericidal activity of ciprofloxacin against amikacin- and cefotaxime-resistant gram-negative bacilli and methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1986 Jun;29(6):1098–1100. doi: 10.1128/aac.29.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):688–691. doi: 10.1128/aac.27.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. In vitro comparison of A-56619, A-56620, amifloxacin, ciprofloxacin, enoxacin, norfloxacin, and ofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):325–326. doi: 10.1128/aac.29.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P. The emergence of methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):440–442. doi: 10.7326/0003-4819-97-3-440. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]


