Abstract
BACKGROUND—Cigarette smoking was shown to delay gastric ulcer healing and reduce synthesis of mucus, which is important for gastric ulcer protection and healing. Polyamines are important in these processes. AIMS—To study the effects of cigarette smoking on the synthesis of mucus and to investigate if such an effect is acting by interference with the polyamine pathway. METHODS—Gastric mucosal ornithine decarboxylase activity, mucous secreting layer thickness, and ulcer size were determined after different concentrations of cigarette smoke exposure (0, 2, or 4%) in intact animals and animals with ulcers. Synthesis of mucus and ornithine decarboxylase activity and mRNA expression were also assessed in cigarette smoke extract treated MKN-28 cells. RESULTS—Exposure to cigarette smoke significantly reduced the thickness of the mucous secreting layer and gastric mucosal ornithine decarboxylase activity in animals with or without ulcers. Spermidine not only reversed inhibition of mucus synthesis in both intact and ulcer bearing animals but also reversed the delay in ulcer healing. Cigarette smoke extract significantly reduced mucus synthesis and ornithine decarboxylase activity but not its mRNA expression in MKN-28 cells. The reduction in mucus synthesis was restored by spermidine. CONCLUSIONS—Cigarette smoke and its extract repress mucus synthesis in vivo and in vitro, respectively. Reduction of ornithine decarboxylase activity in gastric mucosa is closely associated with this effect. Keywords: mucus; ornithine decarboxylase; cigarette smoking; ulcers
Full Text
The Full Text of this article is available as a PDF (173.6 KB).
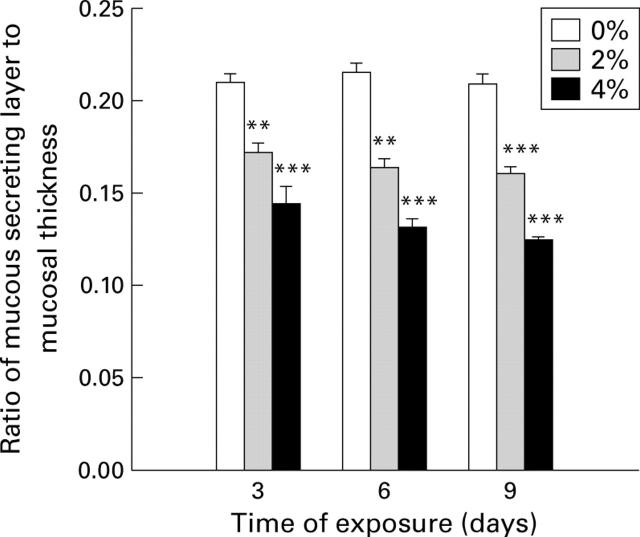
Figure 1 .
Effect of exposure to cigarette smoke on the mucous secreting layer in intact rats. Rats were exposed to cigarette smoke (0, 2, or 4%) for one hour each day for three, six, or nine days. Values are mean (SEM) of 7-8 rats. **p<0.01, ***p<0.001 v 0% exposure.
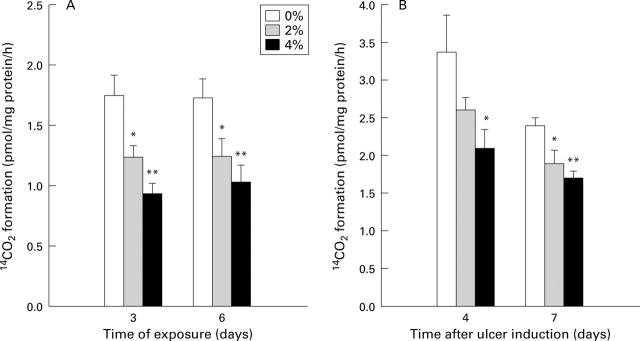
Figure 2 .
Effect of exposure to cigarette smoke on gastric mucosal ornithine decarboxylase (ODC) activity in (A) intact and (B) ulcerated rats. ODC activity was determined by the amount of 14CO2 liberated. Both intact and ulcerated rats (24 hours after ulcer induction) were exposed to cigarette smoke (0, 2, or 4%) for one hour each day for three or six days. ODC activity was measured in the gastric mucosa. Values are mean (SEM) of 7-8 rats. *p<0.05, **p<0.01 v 0% exposure.
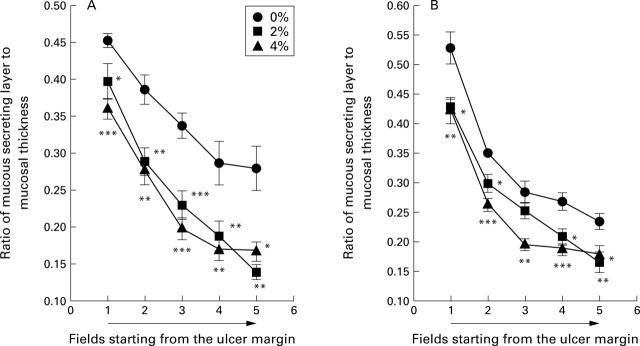
Figure 3 .
Effect of exposure to cigarette smoke on the mucous secreting layer in rats, four (A) and seven (B) days after ulcer induction. Twenty four hours after ulcer induction, rats were exposed to cigarette smoke (0, 2, or 4%) for one hour each day for three or six days. Values are mean (SEM) of 10-12 rats. *p<0.05, **p<0.01, ***p<0.001 v 0% exposure.
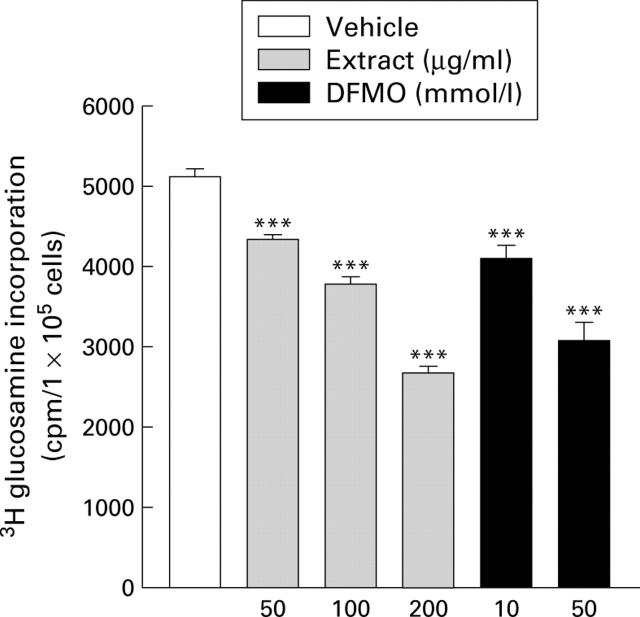
Figure 4 .
Effects of cigarette smoke extract and DL-α-difluoromethyl-ornithine (DFMO) on mucus synthesis in MKN-28 cells. Cells were incubated with cigarette smoke extract, DFMO, or vehicle for six hours. Mucus synthesis was determined by the amount of [3H] glucosamine incorporation. Values are mean (SEM) of six samples. ***p<0.001 v vehicle.
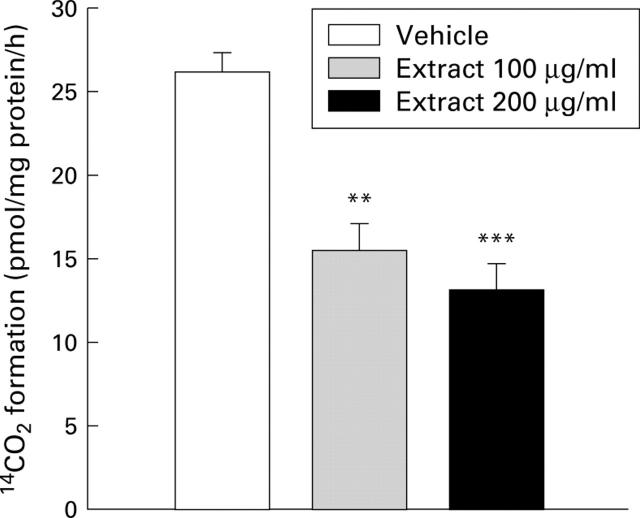
Figure 5 .
Effect of cigarette smoke extract on ornithine decarboxylase (ODC) activity in MKN-28 cells. Cells were incubated with cigarette smoke extract or vehicle for six hours. ODC activity was determined by the amount of 14CO2 liberated. Values are mean (SEM) of six samples. **p<0.01, ***p<0.001 v vehicle.
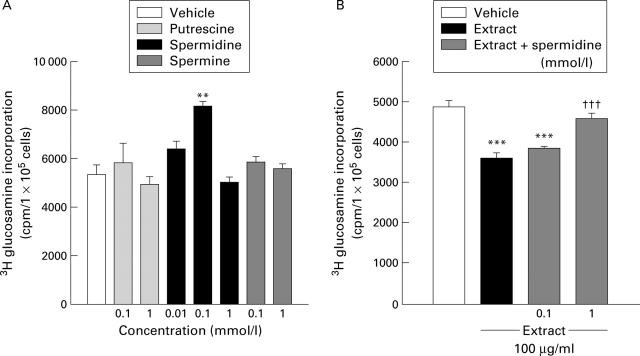
Figure 6 .
Effects of polyamines on mucus synthesis in MKN-28 cells in the absence (A) and presence (B) of cigarette smoke extract. Cells were incubated with vehicle, polyamines, cigarette smoke extract, or a combination of spermidine and smoke extract for six hours. Mucus synthesis was determined by the amount of [3H] glucosamine incorporation. Values are mean (SEM) of six samples. **p<0.01, **p<0.001 v vehicle; †††p <0.001 v cigarette smoke extract alone.
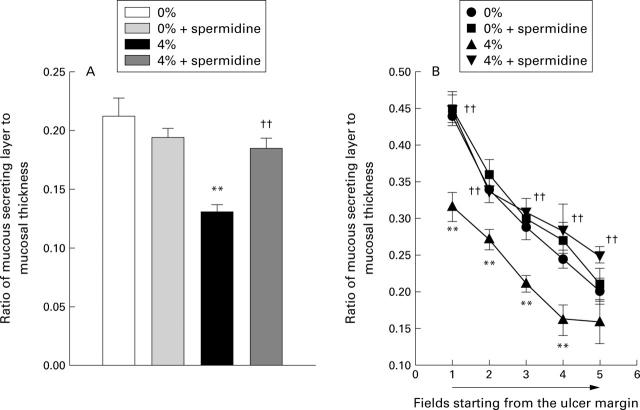
Figure 7 .
Effect of spermidine on inhibition of the mucous secreting layer induced by cigarette smoking in intact (A) and ulcerated (B) rats. Both intact and ulcerated rats (24 hours after ulcer induction) were exposed to cigarette smoke (0, 2, or 4%) for one hour each day for three days. Spermidine was administered intragastrically (100 mg/kg) before each exposure to cigarette smoke. Values are mean (SEM) of 7-8 rats. **p<0.01 v 0% exposure; ††p<0.01 v 4% alone.
Figure 8 .
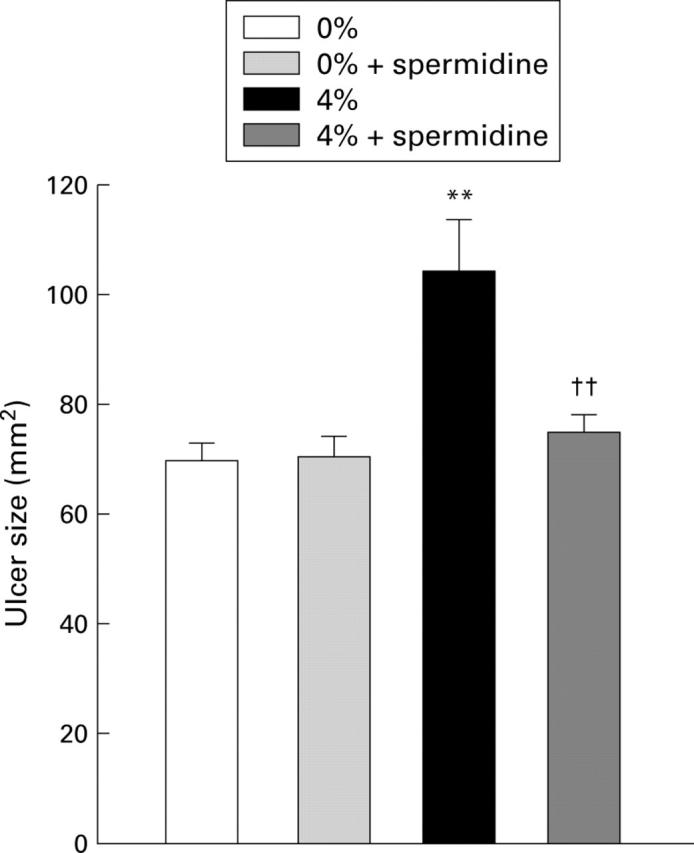
Effect of spermidine on the delay in ulcer healing induced by cigarette smoking, four days after ulcer induction. Twenty four hours after ulcer induction rats were exposed to cigarette smoke (0, 2, or 4%) for one hour each day for three days. Spermidine was administered intragastrically (100 mg/kg) before each exposure. Values are mean (SEM) of 7-8 rats. **p<0.01 v 0% exposure; ††p<0.01 v 4% alone.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Hutton D. A., Leonard A. J., Pearson J. P., Sellers L. A. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1986;125:71–78. doi: 10.3109/00365528609093820. [DOI] [PubMed] [Google Scholar]
- Brown J. F., Hanson P. J., Whittle B. J. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur J Pharmacol. 1992 Nov 13;223(1):103–104. doi: 10.1016/0014-2999(92)90824-n. [DOI] [PubMed] [Google Scholar]
- Brzozowski T., Konturek S. J., Majka J., Dembinski A., Drozdowicz D. Epidermal growth factor, polyamines, and prostaglandins in healing of stress-induced gastric lesions in rats. Dig Dis Sci. 1993 Feb;38(2):276–283. doi: 10.1007/BF01307544. [DOI] [PubMed] [Google Scholar]
- Chow J. Y., Ma L., Cho C. H. An experimental model for studying passive cigarette smoking effects on gastric ulceration. Life Sci. 1996 May 24;58(26):2415–2422. doi: 10.1016/0024-3205(96)00245-7. [DOI] [PubMed] [Google Scholar]
- Chow J. Y., Ma L., Zhu M., Cho C. H. The potentiating actions of cigarette smoking on ethanol-induced gastric mucosal damage in rats. Gastroenterology. 1997 Oct;113(4):1188–1197. doi: 10.1053/gast.1997.v113.pm9322514. [DOI] [PubMed] [Google Scholar]
- DOLL R., JONES F. A., PYGOTT F. Effect of smoking on the production and maintenance of gastric and duodenal ulcers. Lancet. 1958 Mar 29;1(7022):657–662. doi: 10.1016/s0140-6736(58)91083-3. [DOI] [PubMed] [Google Scholar]
- Dai S., Ogle C. W., Cho C. H. Effects of carbenoxolone sodium on gastric and duodenal mucus synthesis in mice. Pharmacology. 1986;33(1):58–60. doi: 10.1159/000138201. [DOI] [PubMed] [Google Scholar]
- Hollanders D., Morrissey S. M. Histochemistry of gastric mucins in smokers and non-smokers. Br J Clin Pract. 1986 Feb;40(2):74–77. [PubMed] [Google Scholar]
- Hyttinen I. M., Halmekytö M., Alhonen L., Jänne J. Levels of ornithine decarboxylase genomic sequences, heterogeneous nuclear RNA and mRNA in human myeloma cells resistant to alpha-difluoromethylornithine. Biochem J. 1991 Sep 15;278(Pt 3):871–874. doi: 10.1042/bj2780871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata F., Leung F. W. Tobacco cigarette smoke aggravates gastric ulcer in rats by attenuation of ulcer margin hyperemia. Am J Physiol. 1995 Jan;268(1 Pt 1):G153–G160. doi: 10.1152/ajpgi.1995.268.1.G153. [DOI] [PubMed] [Google Scholar]
- Jordan N., Newton J., Pearson J., Allen A. A novel method for the visualization of the in situ mucus layer in rat and man. Clin Sci (Lond) 1998 Jul;95(1):97–106. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kato I., Nomura A. M., Stemmermann G. N., Chyou P. H. A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am J Epidemiol. 1992 Mar 1;135(5):521–530. doi: 10.1093/oxfordjournals.aje.a116319. [DOI] [PubMed] [Google Scholar]
- Kelly S. M., Hunter J. O. Epidermal growth factor stimulates synthesis and secretion of mucus glycoproteins in human gastric mucosa. Clin Sci (Lond) 1990 Nov;79(5):425–427. doi: 10.1042/cs0790425. [DOI] [PubMed] [Google Scholar]
- Konturek J. W., Bielanski W., Konturek S. J., Bogdal J., Oleksy J. Distribution and release of epidermal growth factor in man. Gut. 1989 Sep;30(9):1194–1200. doi: 10.1136/gut.30.9.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek J. W., Brzozowski T., Konturek S. J. Epidermal growth factor in protection, repair, and healing of gastroduodenal mucosa. J Clin Gastroenterol. 1991;13 (Suppl 1):S88–S97. doi: 10.1097/00004836-199112001-00015. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Konturek P. K., Majka J., Dembiński A. Role of salivary glands and epidermal growth factor (EGF) in gastric secretion and mucosal integrity in rats exposed to stress. Regul Pept. 1991 Feb 1;32(2):203–215. doi: 10.1016/0167-0115(91)90047-k. [DOI] [PubMed] [Google Scholar]
- Korman M. G., Hansky J., Eaves E. R., Schmidt G. T. Influence of cigarette smoking on healing and relapse in duodenal ulcer disease. Gastroenterology. 1983 Oct;85(4):871–874. [PubMed] [Google Scholar]
- Korman M. G., Shaw R. G., Hansky J., Schmidt G. T., Stern A. I. Influence of smoking on healing rate of duodenal ulcer in response to cimetidine or high-dose antacid. Gastroenterology. 1981 Jun;80(6):1451–1453. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamont J. T., Ventola A. S., Maull E. A., Szabo S. Cysteamine and prostaglandin F2 beta stimulate rat gastric mucin release. Gastroenterology. 1983 Feb;84(2):306–313. [PubMed] [Google Scholar]
- Li K. M. Effect of zinc sulphate on acetic acid-induced gastric ulceration in rats. J Pharm Pharmacol. 1990 Sep;42(9):657–659. doi: 10.1111/j.2042-7158.1990.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Luk I. S., Ho J., Wong W. M., Yuen S. T., Luk C. T., Cho C. H. Influence of chronic nicotine intake and acute ethanol challenge on gastric mucus level and blood flow in rabbits. Digestion. 1994;55(6):399–404. doi: 10.1159/000201172. [DOI] [PubMed] [Google Scholar]
- Ma L., Chow J. Y., Cho C. H. Cigarette smoking delays ulcer healing: role of constitutive nitric oxide synthase in rat stomach. Am J Physiol. 1999 Jan;276(1 Pt 1):G238–G248. doi: 10.1152/ajpgi.1999.276.1.G238. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pullan R. D., Thomas G. A., Rhodes M., Newcombe R. G., Williams G. T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994 Mar;35(3):353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek J., Bilski J., Murty V. L., Slomiany A., Slomiany B. L. Role of salivary epidermal growth factor in the maintenance of physicochemical characteristics of oral and gastric mucosal mucus coat. Biochem Biophys Res Commun. 1988 May 16;152(3):1421–1427. doi: 10.1016/s0006-291x(88)80444-3. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Laszewicz W., Slomiany A. In vitro inhibition of peptic degradation of porcine gastric mucus glycoprotein by sucralfate. Scand J Gastroenterol. 1985 Sep;20(7):857–860. doi: 10.3109/00365528509088835. [DOI] [PubMed] [Google Scholar]
- Sontag S., Graham D. Y., Belsito A., Weiss J., Farley A., Grunt R., Cohen N., Kinnear D., Davis W., Archambault A. Cimetidine, cigarette smoking, and recurrence of duodenal ulcer. N Engl J Med. 1984 Sep 13;311(11):689–693. doi: 10.1056/NEJM198409133111101. [DOI] [PubMed] [Google Scholar]
- Szabo S., Hollander D. Pathways of gastrointestinal protection and repair: mechanisms of action of sucralfate. Am J Med. 1989 Jun 9;86(6A):23–31. doi: 10.1016/0002-9343(89)90153-8. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Okabe S. Roles of extracellular Ca++ and calmodulin in roxatidine-stimulated secretion and synthesis of mucus by cultured rabbit gastric mucosal cells. J Pharmacol Exp Ther. 1998 Jan;284(1):37–42. [PubMed] [Google Scholar]
- Tsujikawa T., Bamba T., Hosoda S. The trophic effect of epidermal growth factor on morphological changes and polyamine metabolism in the small intestine of rats. Gastroenterol Jpn. 1990 Jun;25(3):328–334. doi: 10.1007/BF02779446. [DOI] [PubMed] [Google Scholar]
- Tsukimi Y., Okabe S. Validity of kissing gastric ulcers induced in rats for screening of antiulcer drugs. J Gastroenterol Hepatol. 1994;9 (Suppl 1):S60–S65. doi: 10.1111/j.1440-1746.1994.tb01304.x. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Granger D. N. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996 May;10(7):731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- Wojciechowski K., Trzeciak L., Konturek S. J., Ostrowski J. Inhibition of acid secretory response and induction of ornithine decarboxylase and its mRNA by TGF alpha and EGF in isolated rat gastric glands. Regul Pept. 1995 Mar 7;56(1):1–8. doi: 10.1016/0167-0115(95)00121-q. [DOI] [PubMed] [Google Scholar]
- Wong S. H., Ogle C. W., Cho C. H. The influence of chronic or acute nicotine pretreatment on ethanol-induced gastric ulceration in the rat. J Pharm Pharmacol. 1986 Jul;38(7):537–540. doi: 10.1111/j.2042-7158.1986.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kasuga S., Hirao Y., Fuwa T., Nakagawa S. Effect of biosynthetic human epidermal growth factor on the synthesis and secretion of mucin glycoprotein from primary culture of rabbit fundal mucosa cells. In Vitro Cell Dev Biol. 1987 Jul;23(7):460–464. doi: 10.1007/BF02628415. [DOI] [PubMed] [Google Scholar]