Abstract
OBJECTIVE—To examine the value of universal antenatal screening for hepatitis C virus (HCV) infection among an inner London population, with regard to prevalence, uptake, and acceptability of testing, and identification of new cases. DESIGN—Serum analysis for antibodies against HCV in pregnant women following informed consent ("opt out" policy). Samples positive for HCV antibodies were tested for the presence of HCV RNA by polymerase chain reaction. Information on hepatitis C was provided for all women. Acceptability of antenatal HCV testing and identification of risk factors for infection were assessed through the use of questionnaires randomly distributed among a cohort of 300 pregnant women. SETTING—Antenatal clinics at St Mary's Hospital, London, serving a multiethnic population. SUBJECTS—A total of 4825 pregnant women booking for antenatal care between November 1997 and April 1999. RESULTS—The overall prevalence of anti-HCV was 0.8% and HCV viraemia was 0.6%. Ninety eight per cent of samples (n=4729) were tested; 0.2% of women had a false positive result. In 207 women who completed a questionnaire regarding our testing policy, 84% made a positive decision to be tested for anti-HCV and 92% said that HCV testing should be offered to all pregnant women. The majority (22/32—69%) of HCV infected women were newly diagnosed and although HCV positive women were significantly more likely to have a history of drug abuse, most (16/22—73%) new cases had no identified risk factors for HCV infection at booking. CONCLUSION—The prevalence of anti-HCV in an inner London multiethnic antenatal population is high (0.8%). Routine screening for HCV is acceptable to pregnant women. The majority of women diagnosed during their current pregnancy would not have been identified as HCV infected by epidemiological risk factors at the time of booking. Keywords: hepatitis C; pregnancy; transmission; screening
Full Text
The Full Text of this article is available as a PDF (113.2 KB).
Figure 1 .
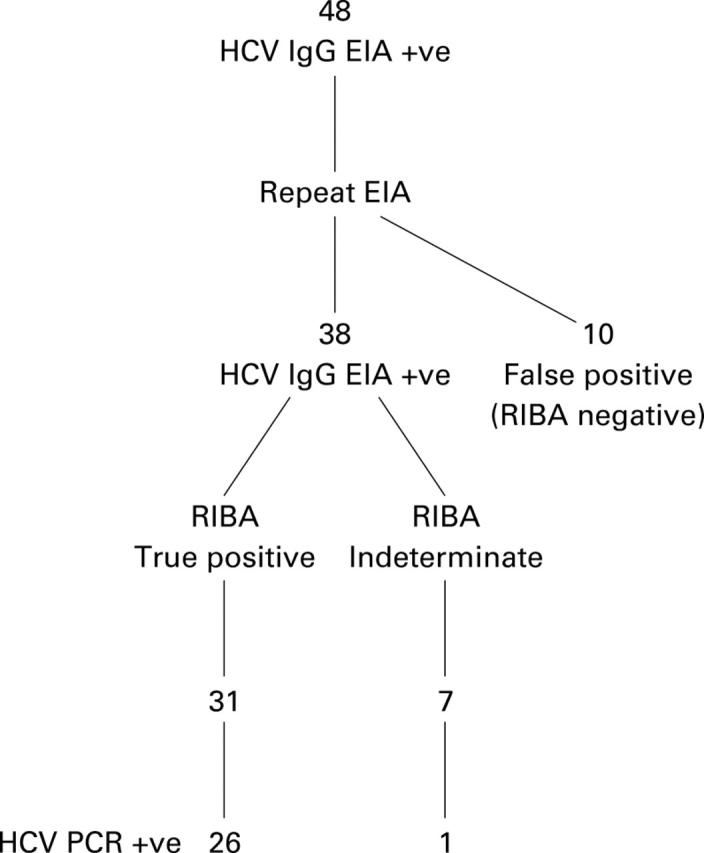
Outcome of testing for hepatitis C virus (HCV) in women with positive antibodies at screening.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter M. J., Sampliner R. E. Hepatitis C: and miles to go before we sleep. N Engl J Med. 1989 Nov 30;321(22):1538–1540. doi: 10.1056/NEJM198911303212208. [DOI] [PubMed] [Google Scholar]
- Boxall E., Skidmore S., Evans C., Nightingale S. The prevalence of hepatitis B and C in an antenatal population of various ethnic origins. Epidemiol Infect. 1994 Dec;113(3):523–528. doi: 10.1017/s0950268800068539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Floreani A., Paternoster D., Zappala F., Cusinato R., Bombi G., Grella P., Chiaramonte M. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996 Apr;103(4):325–329. doi: 10.1111/j.1471-0528.1996.tb09736.x. [DOI] [PubMed] [Google Scholar]
- Foster G. R., Goldin R. D., Main J., Murray-Lyon I., Hargreaves S., Thomas H. C. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ. 1997 Aug 23;315(7106):453–458. doi: 10.1136/bmj.315.7106.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Manzini P., Saracco G., Cerchier A., Riva C., Musso A., Ricotti E., Palomba E., Scolfaro C., Verme G., Bonino F. Human immunodeficiency virus infection as risk factor for mother-to-child hepatitis C virus transmission; persistence of anti-hepatitis C virus in children is associated with the mother's anti-hepatitis C virus immunoblotting pattern. Hepatology. 1995 Feb;21(2):328–332. [PubMed] [Google Scholar]
- McHutchison J. G., Gordon S. C., Schiff E. R., Shiffman M. L., Lee W. M., Rustgi V. K., Goodman Z. D., Ling M. H., Cort S., Albrecht J. K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998 Nov 19;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- Pipan C., Amici S., Astori G., Ceci G. P., Botta G. A. Vertical transmission of hepatitis C virus in low-risk pregnant women. Eur J Clin Microbiol Infect Dis. 1996 Feb;15(2):116–120. doi: 10.1007/BF01591483. [DOI] [PubMed] [Google Scholar]
- Spencer J. D., Latt N., Beeby P. J., Collins E., Saunders J. B., McCaughan G. W., Cossart Y. E. Transmission of hepatitis C virus to infants of human immunodeficiency virus-negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. J Viral Hepat. 1997;4(6):395–409. doi: 10.1046/j.1365-2893.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- Zanetti A. R., Tanzi E., Paccagnini S., Principi N., Pizzocolo G., Caccamo M. L., D'Amico E., Cambiè G., Vecchi L. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995 Feb 4;345(8945):289–291. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]


