Abstract
BACKGROUND AND AIMS—Matrix metalloproteinases (MMPs) are implicated in the tissue destruction associated with inflammatory diseases. Proctocolectomy with ileo-anal pouch (IAP) anastomosis is associated with pouchitis, particularly in patients with ulcerative colitis (UC). The aim of this study was to quantify MMP-1 and MMP-2 in inflamed and uninflamed pouches of patients with UC compared with those with active UC. IAP patients with familial adenomatous polyposis (FAP) served as controls. METHODS—Biopsies were taken from 33 patients with IAP (UC, n=25; FAP, n=8) and from 10 UC patients. MMP-1 and MMP-2 were quantified using sandwich enzyme linked immunosorbent assays. In addition, northern and western blotting and in situ hybridisation experiments were performed. RESULTS—In pouchitis (n=11), MMP-1 and MMP-2 concentrations were increased compared with uninflamed pouches of patients with UC (n=14) or FAP (n=8) (MMP-1 17.7 ng/mg protein v 7.8 (UC) v 7.6 (FAP), p⩽0.05; MMP-2 16.4 v 9.5 (UC) v 6.3 (FAP), p⩽0.05). Western and northern blots revealed increased MMP-1 and MMP-2 protein and transcript concentrations in inflamed pouches. Mesenchymal cells were identified as major producers of MMP-1 and MMP-2 in pouchitis. A similar increase in MMPs was observed in tissues of patients with active UC. CONCLUSIONS—Our results support the hypothesis that MMPs are involved in mucosal destruction and crypt hyperplasia, as seen in pouchitis. Keywords: ileo-anal pouch; matrix metalloproteinases; pouchitis
Full Text
The Full Text of this article is available as a PDF (229.4 KB).
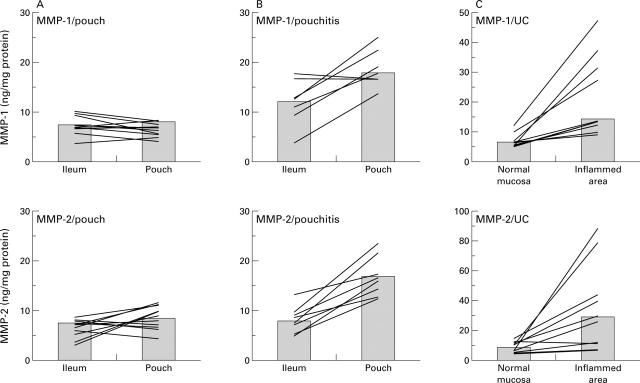
Figure 1 .
MMP-1 and MMP-2 concentrations in uninflamed pouches, in pouchitis, and in active ulcerative colitis (UC). Mucosal biopsies were taken from ileo-anal pouches and the adjacent ileum from individual patients without (A) (n=10) or with pouchitis (B) (n=7) and from patients with active UC (C) (n = 10). For technical reasons it was not possible to take ileal biopsies in one patient with pouchitis and four patients with uninflamed pouches. MMP-1 and MMP-2 concentrations were determined as described in material and methods. Columns represent the median of all patients; lines connect intraindividual MMP-1 and MMP-2 concentrations.
Figure 2 .
Concentrations of MMP-1 and MMP-2 after treatment of pouchitis. Mucosal biopsies were taken from ileo-anal pouches from individual patients (n=7) with pouchitis before and after treatment with metronidazole for two weeks, and analysed for MMP-1 and MMP-2 concentrations. Lines connect the follow up values for MMP concentrations before and after normalisation of clinical and endoscopic signs of inflammation; bars represent median of all patients. In one patient (*), endoscopic follow up after treatment did not show a significant improvement in mucosal inflammation. However, clinical and histological parameters improved.
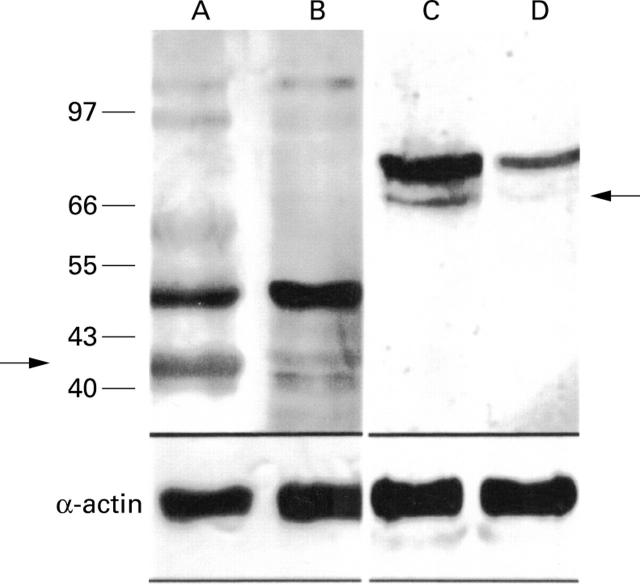
Figure 3 .
Western blot analysis of MMP-1 and MMP-2 expression. Western blotting revealed increased concentrations of MMP proenzymes in pouchitis. Lanes A and C, pouchitis; lanes B and D, uninflamed pouch. Lanes A and B, MMP-1; lanes C and D, MMP-2 (arrows indicate the 42 kDa MMP-1 and the 66 kDa MMP-2). The lower part of the figure shows results of western blot analysis using an α-actin specific antibody to demonstrate equal protein load on each lane.
Figure 4 .
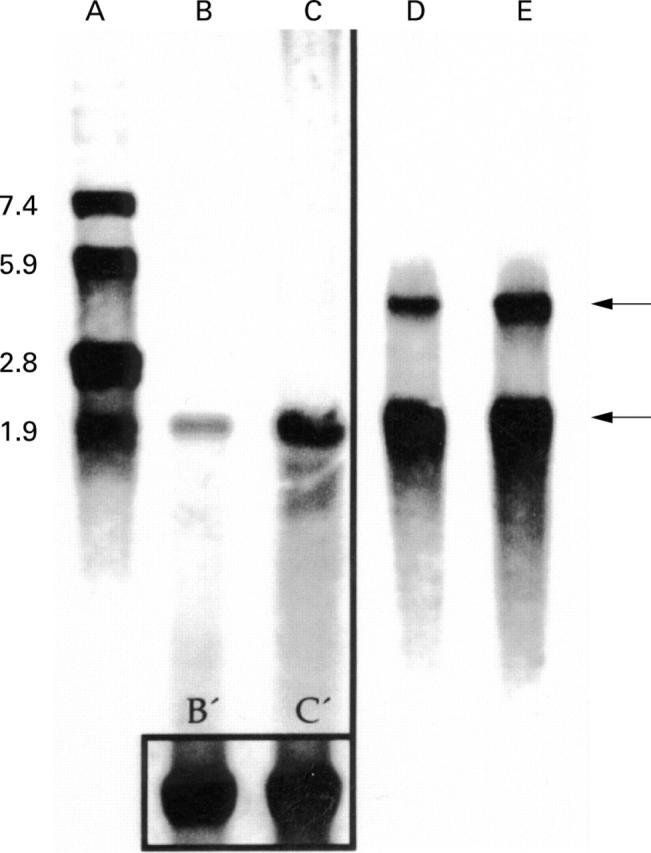
Northern blot analysis of MMP-1- and MMP-2 in mucosal biopsies from patients with pouchitis. Northern blot analysis of MMP-1 (lanes B, C) and MMP-2 (lanes D and E, upper arrow 3.1 kB) and β-actin RNA (lower arrow 1.7 kB). The box in the lower part of lanes B and C represents β-actin RNA of B and C. Lane A represents the RNA bp standard. mRNA was extracted from biopsies from the adjacent uninflamed ileum serving as an internal control (lanes B and D) and inflamed pouch (lanes C and E). A representative example of one of five patients is shown.
Figure 5 .
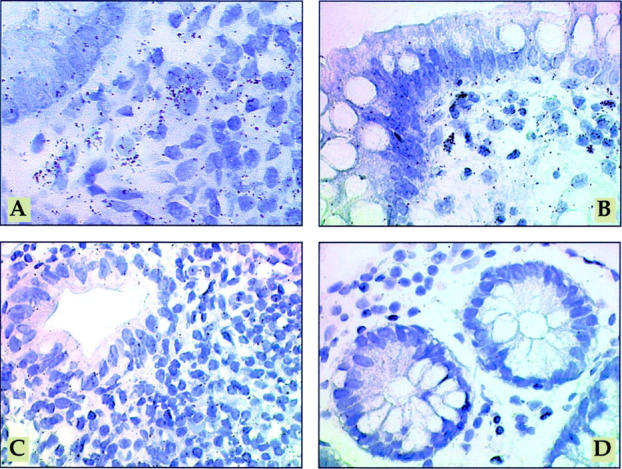
Mesenchymal cells are major producers of MMP-1 and MMP-2 in pouchitis. In situ hybridisation with [35S] labelled MMP-1 antisense RNA probe (A) or [35S] labelled MMP-2 antisense RNA probe (B) in pouchitis. In inflamed pouches a large number of strongly labelled cells is visible in the lamina propria, mainly directly underneath the epithelium (A, B). [35S] labelled MMP-1 sense RNA probe (C) in pouchitis, demonstrating the background signal. In situ hybridisation with [35S] labelled MMP-2 antisense RNA probe (D) on ileum tissue with mild inflammation. In the uninflamed ileum grains are localised over mesenchymal cells of the lamina propria, predominantly underneath the epithelium. However, transcript levels were near the threshold of detection in most cases. Exposure time in all sections was 28 days. Original magnification ×600.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airola K., Vaalamo M., Reunala T., Saarialho-Kere U. K. Enhanced expression of interstitial collagenase, stromelysin-1, and urokinase plasminogen activator in lesions of dermatitis herpetiformis. J Invest Dermatol. 1995 Aug;105(2):184–189. doi: 10.1111/1523-1747.ep12317093. [DOI] [PubMed] [Google Scholar]
- Bailey C. J., Hembry R. M., Alexander A., Irving M. H., Grant M. E., Shuttleworth C. A. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994 Feb;47(2):113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh M. D., Perry M. J., Hollander A. P., Davies D. R., Cross S. S., Lobo A. J., Taylor C. J., Evans G. S. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999 Oct;117(4):814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Browne K. A., Green P. A., Jaspar J. M., Maini R. N., Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-alpha (cA2) therapy. Br J Rheumatol. 1997 Jun;36(6):643–650. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Ramsey S., Hazleman B. L., Cawston T. E. The measurement of collagenase, tissue inhibitor of metalloproteinases (TIMP), and collagenase-TIMP complex in synovial fluids from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):372–379. doi: 10.1002/art.1780360313. [DOI] [PubMed] [Google Scholar]
- Conway J. G., Wakefield J. A., Brown R. H., Marron B. E., Sekut L., Stimpson S. A., McElroy A., Menius J. A., Jeffreys J. J., Clark R. L. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med. 1995 Aug 1;182(2):449–457. doi: 10.1084/jem.182.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum S., Bauer U., Foss H. D., Schuppan D., Stein H., Riecken E. O., Ullrich R. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut. 1999 Jan;44(1):17–25. doi: 10.1136/gut.44.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. F., Willey A., Adams J., Yager D., Diegelmann R. F. Interleukin 1 beta down-regulates collagen and augments collagenase expression in human intestinal smooth muscle cells. Gastroenterology. 1996 Feb;110(2):344–350. doi: 10.1053/gast.1996.v110.pm8566579. [DOI] [PubMed] [Google Scholar]
- Grant G. M., Giambernardi T. A., Grant A. M., Klebe R. J. Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol. 1999 Apr;18(2):145–148. doi: 10.1016/s0945-053x(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Morales A. R., Romanelli R., Woessner J. F., Jr Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol. 1996 May;148(5):1639–1648. [PMC free article] [PubMed] [Google Scholar]
- Günther U., Schuppan D., Bauer M., Matthes H., Stallmach A., Schmitt-Gräff A., Riecken E. O., Herbst H. Fibrogenesis and fibrolysis in collagenous colitis. Patterns of procollagen types I and IV, matrix-metalloproteinase-1 and -13, and TIMP-1 gene expression. Am J Pathol. 1999 Aug;155(2):493–503. doi: 10.1016/S0002-9440(10)65145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet E. H., Docherty A. J., Beertsen W., Everts V. Collagen breakdown in soft connective tissue explants is associated with the level of active gelatinase A (MMP-2) but not with collagenase. Matrix Biol. 1999 Aug;18(4):373–380. doi: 10.1016/s0945-053x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Some molecular mechanisms of glucocorticoid action. Br J Rheumatol. 1993 May;32 (Suppl 2):3–5. doi: 10.1093/rheumatology/32.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Matthes H., Herbst H., Schuppan D., Stallmach A., Milani S., Stein H., Riecken E. O. Cellular localization of procollagen gene transcripts in inflammatory bowel diseases. Gastroenterology. 1992 Feb;102(2):431–442. doi: 10.1016/0016-5085(92)90087-f. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Grappone C., Pellegrini G., Pinzani M., Casini A., Calabró A., Ciancio G., Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994 Mar;144(3):528–537. [PMC free article] [PubMed] [Google Scholar]
- Orzechowski H. D., Beckenbach C., Herbst H., Stölzel U., Riecken E. O., Stallmach A. Expression of CD44v6 is associated with cellular dysplasia in colorectal epithelial cells. Eur J Cancer. 1995 Nov;31A(12):2073–2079. doi: 10.1016/0959-8049(95)00452-1. [DOI] [PubMed] [Google Scholar]
- Parks A. G., Nicholls R. J. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978 Jul 8;2(6130):85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. T., Bain I., Youngs D., Keighley M. R. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995 Aug;38(8):831–837. doi: 10.1007/BF02049839. [DOI] [PubMed] [Google Scholar]
- Pender S. L., Breese E. J., Günther U., Howie D., Wathen N. C., Schuppan D., MacDonald T. T. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998 Sep;115(3):573–583. doi: 10.1016/s0016-5085(98)70136-2. [DOI] [PubMed] [Google Scholar]
- Pender S. L., Fell J. M., Chamow S. M., Ashkenazi A., MacDonald T. T. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998 Apr 15;160(8):4098–4103. [PubMed] [Google Scholar]
- Pender S. L., Tickle S. P., Docherty A. J., Howie D., Wathen N. C., MacDonald T. T. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997 Feb 15;158(4):1582–1590. [PubMed] [Google Scholar]
- Present D. H., Rutgeerts P., Targan S., Hanauer S. B., Mayer L., van Hogezand R. A., Podolsky D. K., Sands B. E., Braakman T., DeWoody K. L. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999 May 6;340(18):1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994 Aug;8(11):823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Vaalamo M., Puolakkainen P., Airola K., Parks W. C., Karjalainen-Lindsberg M. L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996 Feb;148(2):519–526. [PMC free article] [PubMed] [Google Scholar]
- Sandborn W. J. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994 Dec;107(6):1856–1860. doi: 10.1016/0016-5085(94)90832-x. [DOI] [PubMed] [Google Scholar]
- Shepherd N. A., Jass J. R., Duval I., Moskowitz R. L., Nicholls R. J., Morson B. C. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987 Jun;40(6):601–607. doi: 10.1136/jcp.40.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach A., Schäfer F., Hoffmann S., Weber S., Müller-Molaian I., Schneider T., Köhne G., Ecker K. W., Feifel G., Zeitz M. Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchitis. Gut. 1998 Oct;43(4):499–505. doi: 10.1136/gut.43.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson C. J., Talhouk R. S., Alexander C. M., Chin J. R., Clift S. M., Bissell M. J., Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994 May;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. A., Dozois R. R. The J ileal pouch-anal anastomosis. World J Surg. 1987 Dec;11(6):727–734. doi: 10.1007/BF01656595. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Bair M. J., Bauer E. A., Amento E. P. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-gamma. J Biol Chem. 1991 Dec 5;266(34):23477–23482. [PubMed] [Google Scholar]
- Vaalamo M., Karjalainen-Lindsberg M. L., Puolakkainen P., Kere J., Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998 Apr;152(4):1005–1014. [PMC free article] [PubMed] [Google Scholar]
- Weg-Remers S., Schüder G., Zeitz M., Stallmach A. CD44 expression in colorectal cancer. Ann N Y Acad Sci. 1998 Nov 17;859:304–306. doi: 10.1111/j.1749-6632.1998.tb11151.x. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]