Abstract
BACKGROUND—In normal gastric epithelium, MUC5AC is detected in superficial epithelium associated with Lewis type 1 antigens and MUC6 is detected in antral glands with Lewis type 2. Therefore, the stomach constitutes an excellent model to examine the role of glycosyltransferases in determining the specificity of apomucin glycosylation. AIMS—To determine the molecular basis of this association and to examine changes in expression of gastric and intestinal apomucins and their association with Lewis antigens during the gastric carcinogenesis process. METHODS—Fucosyltransferase (FUT1, FUT2, FUT3) and mucin (MUC5AC, MUC6) transcripts were detected using reverse transcription-polymerase chain reaction. Apomucin (MUC2, MUC4, MUC5AC, MUC6) and Lewis antigen (types 1 and 2) expression were analysed using single and double immunohistochemistry and in situ hybridisation. RESULTS—In the normal stomach, FUT1 is exclusively detected associated with MUC6; FUT2 is only detected when MUC5AC is present. This co-regulation is lost in gastric tumours, as is differential expression of MUC5AC and MUC6 in normal gastric epithelial cells. In gastric tumours, especially those with the intestinal phenotype, MUC2 and MUC4 genes are upregulated, and gastric-type and intestinal-type mucins are coexpressed. These changes are early events in the gastric carcinogenesis process, as they are detected in intestinal metaplasia. CONCLUSIONS—The glycosylation pattern found in normal gastric epithelium is dictated by the specific set of fucosyltranferases expressed by the cells rather than by the apomucin sequence. The development of intestinal metaplasia and gastric cancer is associated with the appearance of cellular phenotypes that are absent from normal epithelium. Keywords: fucosyltransferases; gastric carcinogenesis; gastric mucins; Lewis antigens
Full Text
The Full Text of this article is available as a PDF (203.4 KB).
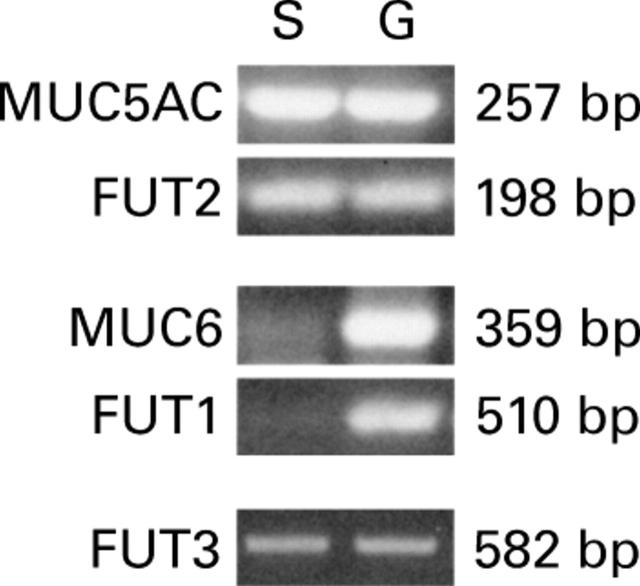
Figure 1 .
Mucin and fucosyltransferase mRNA expression in gastric mucosa scrapings detected by RT-PCR (S, superficial epithelium; G, deep glands). MUC6 and FUT1 were detected only in the deep gland scrapings. MUC5AC and FUT2 were detected in both superficial epithelium and deep glands, suggesting that cells from the superficial epithelium contaminated the deep gland fraction.
Figure 2 .
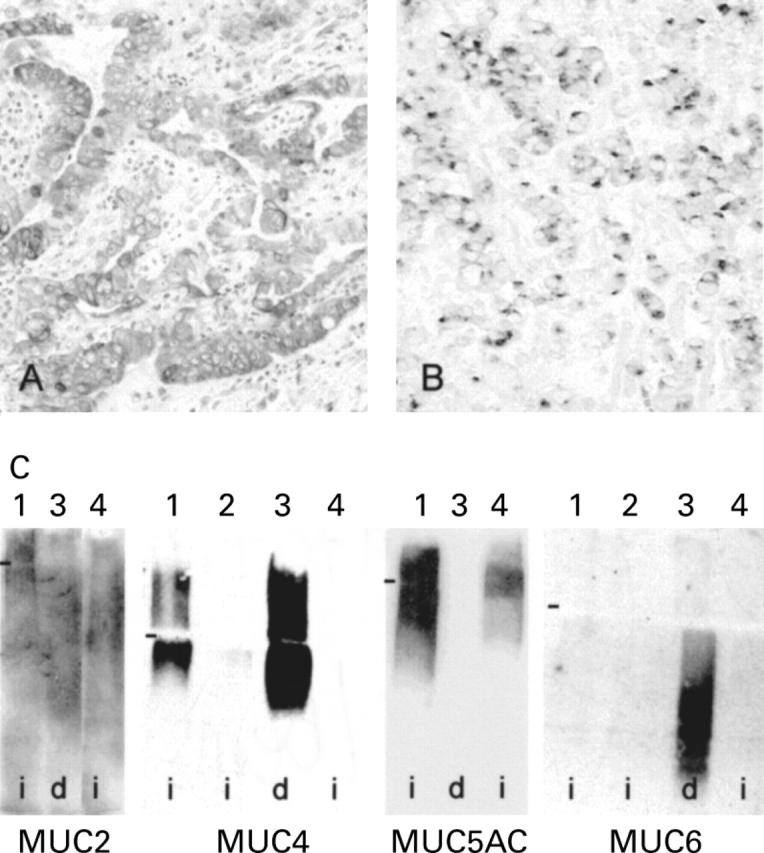
Mucin expression in gastric tumours. (A) MUC4 detected by immunohistochemistry in an intestinal-type tumour. (B) MUC4 expression by in situ hybridisation in a diffuse-type tumour (original magnification: A ×200; B ×100). (C) Coexpression of several apomucin genes in gastric tumours: western blot detection of MUC2, MUC4, MUC5AC, and MUC6 apomucins in lysates of intestinal (i) (1, 2, 4) and diffuse (d) (3) gastric tumours. Nitrocellulose membranes were treated with 100 mM sodium periodate before immunodetection. Several apomucins are detected in the same tumour sample. Markers (−) indicate the borders between running and stacking gels.
Figure 3 .
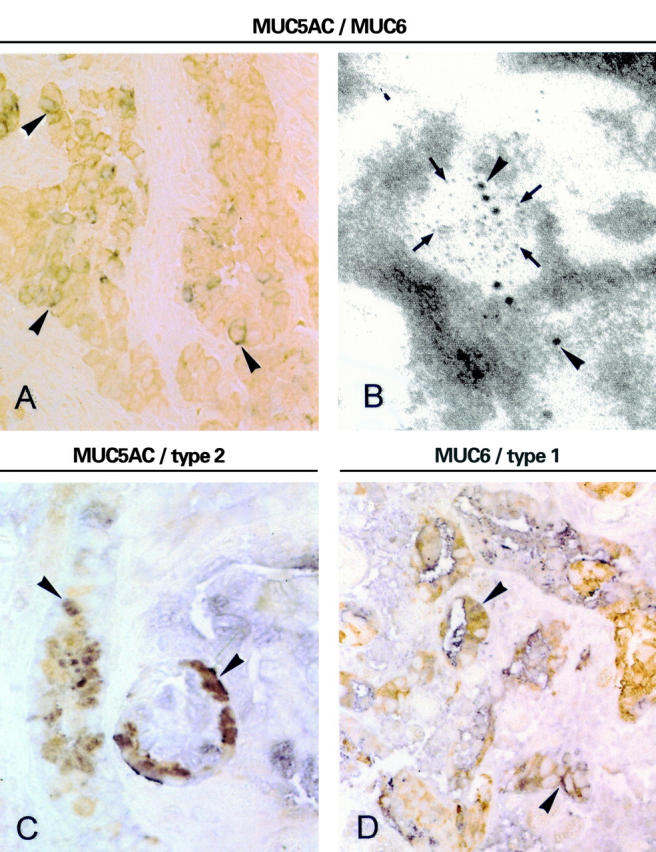
Gastric cancers contain cells displaying abnormal patterns of apomucin/Lewis antigen coexpression. (A) Double labelling immunohistochemical detection of MUC5AC (blue) and MUC6 expression (light brown) cells. Doubly labelled cells display a dark brown colour (original magnification ×400). (B) Double labelling immunoelectron microscopy using antibodies against MUC5AC (CLH2) and MUC6 (anti-MUC6.1) and sections from Lowicryl 4KM embedded tissues. Mouse antibodies were detected using 15 nm gold particles (arrowheads) and rabbit antibodies using 5 nm gold particles (arrows) (original magnification ×63 000). (C) Double labelling immunohistochemical detection of MUC5AC (brown) and Lewis y (blue). (D) Double labelling immunohistochemical detection of MUC6 (brown) and Lewis b (blue). Arrowheads in (A), (C), and (D) indicate coexpression in tumour cells (original magnification ×400).
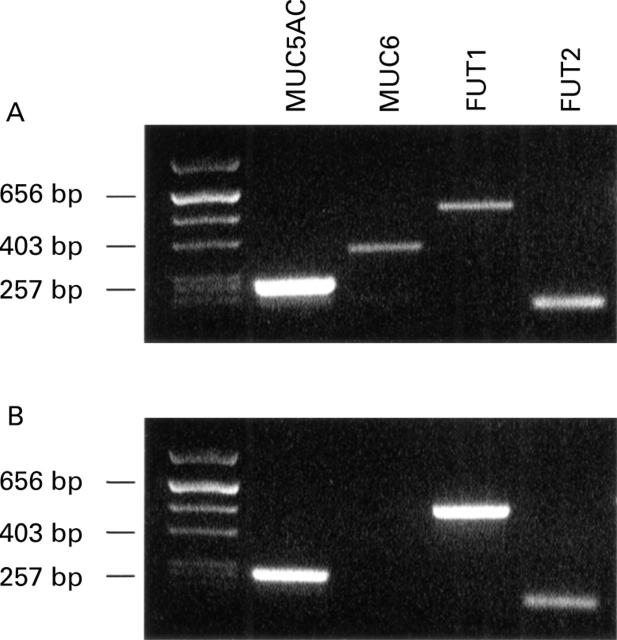
Figure 4 .
RT-PCR analysis of fucosyltransferase (FUT1 and FUT2) and mucin (MUC5AC and MUC6) transcripts in two gastric tumours (A and B). There was no association between the presence of MUC5AC and MUC6, and FUT2 and FUT1 transcripts, respectively.
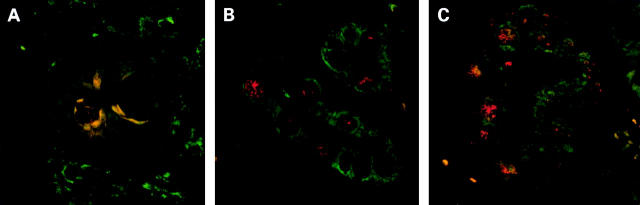
Figure 5 .
Expression of apomucins and Lewis antigens in intestinal metaplasia. Double labelling immunofluorescence detection of: (A) MUC2 (green) and MUC6 (red); (B) MUC4 (green) and Lewis b (red); (C) MUC5AC (green) and Lewis y (red). In incomplete intestinal metaplasia, coexpression of gastric and intestinal apomucins at the single cell level is demonstrated. In addition, the association between apomucin and Lewis antigens present in normal stomach is lost (original magnification: A ×630; B, C ×400).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audie J. P., Janin A., Porchet N., Copin M. C., Gosselin B., Aubert J. P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Berrozpe G., Schaeffer J., Peinado M. A., Real F. X., Perucho M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994 Jul 15;58(2):185–191. doi: 10.1002/ijc.2910580207. [DOI] [PubMed] [Google Scholar]
- Bobek L. A., Tsai H., Biesbrock A. R., Levine M. J. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem. 1993 Sep 25;268(27):20563–20569. [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Bry L., Falk P. G., Midtvedt T., Gordon J. I. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996 Sep 6;273(5280):1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Yan P., Sternberg L., Yunker C. K., Scheiman J. M., Bresalier R. S. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997 Aug;113(2):455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- Cameron H. S., Szczepaniak D., Weston B. W. Expression of human chromosome 19p alpha(1,3)-fucosyltransferase genes in normal tissues. Alternative splicing, polyadenylation, and isoforms. J Biol Chem. 1995 Aug 25;270(34):20112–20122. doi: 10.1074/jbc.270.34.20112. [DOI] [PubMed] [Google Scholar]
- Carrato C., Balague C., de Bolos C., Gonzalez E., Gambus G., Planas J., Perini J. M., Andreu D., Real F. X. Differential apomucin expression in normal and neoplastic human gastrointestinal tissues. Gastroenterology. 1994 Jul;107(1):160–172. doi: 10.1016/0016-5085(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crepin M., Porchet N., Aubert J. P., Degand P. Diversity of the peptide moiety of human airway mucins. Biorheology. 1990;27(3-4):471–484. doi: 10.3233/bir-1990-273-426. [DOI] [PubMed] [Google Scholar]
- De Bolós C., Garrido M., Real F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995 Sep;109(3):723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- Dufosse J., Porchet N., Audie J. P., Guyonnet Duperat V., Laine A., Van-Seuningen I., Marrakchi S., Degand P., Aubert J. P. Degenerate 87-base-pair tandem repeats create hydrophilic/hydrophobic alternating domains in human mucin peptides mapped to 11p15. Biochem J. 1993 Jul 15;293(Pt 2):329–337. doi: 10.1042/bj2930329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea G., Francí C., Gambús G., Lesuffleur T., Zweibaum A., Real F. X. cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells. J Cell Sci. 1993 Jul;105(Pt 3):819–830. doi: 10.1242/jcs.105.3.819. [DOI] [PubMed] [Google Scholar]
- Falk P. G., Bry L., Holgersson J., Gordon J. I. Expression of a human alpha-1,3/4-fucosyltransferase in the pit cell lineage of FVB/N mouse stomach results in production of Leb-containing glycoconjugates: a potential transgenic mouse model for studying Helicobacter pylori infection. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1515–1519. doi: 10.1073/pnas.92.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambús G., de Bolós C., Andreu D., Francí C., Egea G., Real F. X. Detection of the MUC2 apomucin tandem repeat with a mouse monoclonal antibody. Gastroenterology. 1993 Jan;104(1):93–102. doi: 10.1016/0016-5085(93)90840-9. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Kim Y. S. Identification and characterization of the MUC2 (human intestinal mucin) gene 5'-flanking region: promoter activity in cultured cells. Biochem J. 1997 Jul 1;325(Pt 1):259–267. doi: 10.1042/bj3250259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Hanukoglu I., Tanese N., Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. doi: 10.1016/0022-2836(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Ho S. B., Shekels L. L., Toribara N. W., Kim Y. S., Lyftogt C., Cherwitz D. L., Niehans G. A. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995 Jun 15;55(12):2681–2690. [PubMed] [Google Scholar]
- Hovenberg H. W., Davies J. R., Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996 Aug 15;318(Pt 1):319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. J., Ernst L. K., Larsen R. D., Bryant J. G., Robinson J. S., Lowe J. B. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5843–5847. doi: 10.1073/pnas.91.13.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. J., Rouquier S., Giorgi D., Lennon G. G., Lowe J. B. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995 Mar 3;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- Kitagawa H., Paulson J. C. Differential expression of five sialyltransferase genes in human tissues. J Biol Chem. 1994 Jul 8;269(27):17872–17878. [PubMed] [Google Scholar]
- Koda Y., Soejima M., Kimura H. Changing transcription start sites in H-type alpha(1,2)fucosyltransferase gene (FUT1) during differentiation of the human erythroid lineage. Eur J Biochem. 1998 Sep 1;256(2):379–387. doi: 10.1046/j.1432-1327.1998.2560379.x. [DOI] [PubMed] [Google Scholar]
- Koda Y., Soejima M., Kimura H. Structure and expression of H-type GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase gene (FUT1). Two transcription start sites and alternative splicing generate several forms of FUT1 mRNA. J Biol Chem. 1997 Mar 14;272(11):7501–7505. doi: 10.1074/jbc.272.11.7501. [DOI] [PubMed] [Google Scholar]
- Lan M. S., Batra S. K., Qi W. N., Metzgar R. S., Hollingsworth M. A. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem. 1990 Sep 5;265(25):15294–15299. [PubMed] [Google Scholar]
- Laurén P. Histogenesis of intestinal and diffuse types of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;180:160–164. [PubMed] [Google Scholar]
- Lesuffleur T., Porchet N., Aubert J. P., Swallow D., Gum J. R., Kim Y. S., Real F. X., Zweibaum A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993 Nov;106(Pt 3):771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T., Roche F., Hill A. S., Lacasa M., Fox M., Swallow D. M., Zweibaum A., Real F. X. Characterization of a mucin cDNA clone isolated from HT-29 mucus-secreting cells. The 3' end of MUC5AC? J Biol Chem. 1995 Jun 9;270(23):13665–13673. doi: 10.1074/jbc.270.23.13665. [DOI] [PubMed] [Google Scholar]
- Li D., Gallup M., Fan N., Szymkowski D. E., Basbaum C. B. Cloning of the amino-terminal and 5'-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J Biol Chem. 1998 Mar 20;273(12):6812–6820. doi: 10.1074/jbc.273.12.6812. [DOI] [PubMed] [Google Scholar]
- Mentis A., Blackwell C. C., Weir D. M., Spiliadis C., Dailianas A., Skandalis N. ABO blood group, secretor status and detection of Helicobacter pylori among patients with gastric or duodenal ulcers. Epidemiol Infect. 1991 Apr;106(2):221–229. doi: 10.1017/s0950268800048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Egami H., Shibata Y., Sakamoto K., Misumi A., Ogawa M. Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am J Clin Pathol. 1992 Jul;98(1):67–75. doi: 10.1093/ajcp/98.1.67. [DOI] [PubMed] [Google Scholar]
- Ottini L., Palli D., Falchetti M., D'Amico C., Amorosi A., Saieva C., Calzolari A., Cimoli F., Tatarelli C., De Marchis L. Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res. 1997 Oct 15;57(20):4523–4529. [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Reis C. A., David L., Correa P., Carneiro F., de Bolós C., Garcia E., Mandel U., Clausen H., Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999 Mar 1;59(5):1003–1007. [PubMed] [Google Scholar]
- Reis C. A., David L., Nielsen P. A., Clausen H., Mirgorodskaya K., Roepstorff P., Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997 Feb 20;74(1):112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sakamoto J., Furukawa K., Cordon-Cardo C., Yin B. W., Rettig W. J., Oettgen H. F., Old L. J., Lloyd K. O. Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986 Mar;46(3):1553–1561. [PubMed] [Google Scholar]
- Semba S., Yokozaki H., Yamamoto S., Yasui W., Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996 Apr 15;77(8 Suppl):1620–1627. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1620::AID-CNCR30>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]
- Torrado J., Correa P., Ruiz B., Bernardi P., Zavala D., Bara J. Lewis antigen alterations in gastric cancer precursors. Gastroenterology. 1992 Feb;102(2):424–430. doi: 10.1016/0016-5085(92)90086-e. [DOI] [PubMed] [Google Scholar]
- Tytgat K. M., Büller H. A., Opdam F. J., Kim Y. S., Einerhand A. W., Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994 Nov;107(5):1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Ura H., Denno R., Hirata K., Yamaguchi K., Yasoshima T., Shishido T. Close correlation between increased sialyl-Lewisx expression and metastasis in human gastric carcinoma. World J Surg. 1997 Sep;21(7):773–776. doi: 10.1007/s002689900304. [DOI] [PubMed] [Google Scholar]