Abstract
BACKGROUND—The glucagon-like peptides (GLP) 1 and 2 are secreted postprandially from L cells located mainly in the ileum. Both hormones prolong intestinal transit and GLP-2 is intestinotrophic in rodents. Patients with a jejunostomy have poor adaptation, rapid gastric and intestinal transit, and impaired postprandial GLP-2 secretion. Ileum resected short bowel patients with a preserved colon show evidence of functional adaptation and have normal gastric emptying. AIM—To investigate if GLP-1 and GLP-2 contribute to the positive effects of a preserved colon in short bowel patients by measuring circulating levels of GLP-1 and GLP-2 in seven ileum resected short bowel patients with a preserved colon and seven age and sex matched controls. METHODS—GLP-1 and GLP-2 immunoreactivity was measured by specific radioimmunoassays in plasma collected at fasting and at regular intervals 180 minutes after a test meal. RESULTS—Median (25-75%) fasting GLP-2 values were 72 (69-105) pmol/l versus 23 (19-27) pmol/l (p=0.001) and meal stimulated area under the curve was 21 078 (14 811-26 610) min×pmol/l versus 11 150 (7151-12 801) min×pmol/l (p=0.01) in short bowel patients with a preserved colon compared with control subjects. Fasting GLP-1 values were 10 (6-12) pmol/l versus 5 (3-5) pmol/l (p=0.01) and meal stimulated area under the curve was 3418 (2966-6850) min×pmol/l versus 2478 (1929-3199) min×pmol/l (p=0.04), respectively. CONCLUSION—Ileum resected short bowel patients with a preserved colon had elevated fasting plasma concentrations of GLP-1 and GLP-2 and significantly larger meal stimulated areas under the curve compared with age and sex matched controls. Elevated GLP-1 and GLP-2 concentrations may contribute to the positive effects of a preserved colon on intestinal motility and functional adaptation in ileum resected short bowel patients. Keywords: short bowel syndrome; colon; glucagon-like peptides; intestinal adaptation; intestinal transit
Full Text
The Full Text of this article is available as a PDF (139.3 KB).
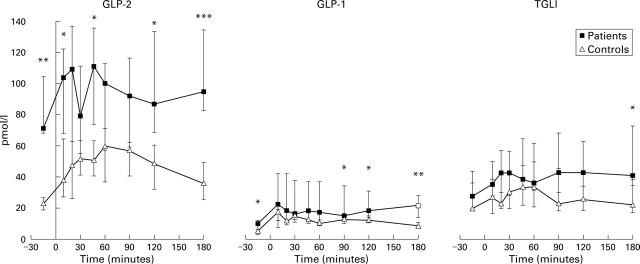
Figure 1 .
Meal stimulated glucagon-like peptides 1 and 2 (GLP-2, GLP-1), and total glucagon-like immunoreactivity (TGLI) responses (pmol/l) in patients and controls. Results are median (25-75%).*p<0.05, **p<0.01,***p<0.001, Mann-Whitney rank sum test, patients versus controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Ferri G. L., Bacarese-Hamilton A. J., Fuessl H. S., Polak J. M., Bloom S. R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985 Nov;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Polak J. M. The hormonal pattern of intestinal adaptation. A major role for enteroglucagon. Scand J Gastroenterol Suppl. 1982;74:93–103. [PubMed] [Google Scholar]
- Carbonnel F., Cosnes J., Chevret S., Beaugerie L., Ngô Y., Malafosse M., Parc R., Le Quintrec Y., Gendre J. P. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996 Jul-Aug;20(4):275–280. doi: 10.1177/0148607196020004275. [DOI] [PubMed] [Google Scholar]
- Cassidy M. M., Lightfoot F. G., Grau L. E., Story J. A., Kritchevsky D., Vahouny G. V. Effect of chronic intake of dietary fibers on the ultrastructural topography of rat jejunum and colon: a scanning electron microscopy study. Am J Clin Nutr. 1981 Feb;34(2):218–228. doi: 10.1093/ajcn/34.2.218. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., James W. P., Wiggins H. S. Role of the colon in ileal-resection diarrhoea. Lancet. 1973 Feb 17;1(7799):344–347. doi: 10.1016/s0140-6736(73)90131-1. [DOI] [PubMed] [Google Scholar]
- Dowling R. H. Small bowel adaptation and its regulation. Scand J Gastroenterol Suppl. 1982;74:53–74. [PubMed] [Google Scholar]
- Drucker D. J., Erlich P., Asa S. L., Brubaker P. L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPEN-Home Artificial Nutrition Working Group. Van Gossum A., Bakker H., De Francesco A., Ladefoged K., Leon-Sanz M., Messing B., Pironi L., Pertkiewicz M., Shaffer J. Home parenteral nutrition in adults: a multicentre survey in Europe in 1993. Clin Nutr. 1996 Apr;15(2):53–59. doi: 10.1016/s0261-5614(96)80019-7. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Townsend C. M., Jr, Uchida T., Thompson J. C. Neurotensin prevents intestinal mucosal hypoplasia in rats fed an elemental diet. Dig Dis Sci. 1992 Mar;37(3):426–431. doi: 10.1007/BF01307738. [DOI] [PubMed] [Google Scholar]
- Frankel W. L., Zhang W., Singh A., Klurfeld D. M., Don S., Sakata T., Modlin I., Rombeau J. L. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994 Feb;106(2):375–380. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Bloom S. R., Polak J. M., Henry K., Dowling R. H. Endocrine tumour in kidney affecting small bowel structure, motility, and absorptive function. Gut. 1971 Oct;12(10):773–782. doi: 10.1136/gut.12.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Lenton W., Ghatei M. A., Adrian T. E., Bloom S. R., Wright N. A. Effects of an elemental diet, inert bulk and different types of dietary fibre on the response of the intestinal epithelium to refeeding in the rat and relationship to plasma gastrin, enteroglucagon, and PYY concentrations. Gut. 1987 Feb;28(2):171–180. doi: 10.1136/gut.28.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Johnsen A. H., Orskov C., Adelhorst K., Thim L., Holst J. J. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000 Jan;21(1):73–80. doi: 10.1016/s0196-9781(99)00176-x. [DOI] [PubMed] [Google Scholar]
- Holst J. J., Bersani M., Johnsen A. H., Kofod H., Hartmann B., Orskov C. Proglucagon processing in porcine and human pancreas. J Biol Chem. 1994 Jul 22;269(29):18827–18833. [PubMed] [Google Scholar]
- Holst J. J. Enteroglucagon. Annu Rev Physiol. 1997;59:257–271. doi: 10.1146/annurev.physiol.59.1.257. [DOI] [PubMed] [Google Scholar]
- Holst J. J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J. 1982 Dec 1;207(3):381–388. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J. Evidence that glicentin contains the entire sequence of glucagon. Biochem J. 1980 May 1;187(2):337–343. doi: 10.1042/bj1870337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994 Dec;107(6):1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Hvidberg A., Nielsen M. T., Hilsted J., Orskov C., Holst J. J. Effect of glucagon-like peptide-1 (proglucagon 78-107amide) on hepatic glucose production in healthy man. Metabolism. 1994 Jan;43(1):104–108. doi: 10.1016/0026-0495(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Izukura M., Evers B. M., Parekh D., Yoshinaga K., Uchida T., Townsend C. M., Jr, Thompson J. C. Neurotensin augments intestinal regeneration after small bowel resection in rats. Ann Surg. 1992 May;215(5):520–527. doi: 10.1097/00000658-199205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A. P., Thompson R. P. Enteral nutrition and the small intestine. Gut. 1994 Dec;35(12):1765–1769. doi: 10.1136/gut.35.12.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B., Hartmann B., Hansen B. S., Thulesen J., Holst J. J., Mortensen P. B. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999 Oct;45(4):559–563. doi: 10.1136/gut.45.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B., Mortensen P. B. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut. 2000 May;46(5):701–706. doi: 10.1136/gut.46.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B., Mortensen P. B. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998 Oct;43(4):478–483. doi: 10.1136/gut.43.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. B., Staun M., Mortensen P. B. Adult patients receiving home parenteral nutrition in Denmark from 1991 to 1996: who will benefit from intestinal transplantation? Scand J Gastroenterol. 1998 Aug;33(8):839–846. doi: 10.1080/00365529850171503. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. B., Staun M., Tjellesen L., Mortensen P. B. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut. 1998 Dec;43(6):763–769. doi: 10.1136/gut.43.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruda M. J., Rolandelli R. H., Bliss D. Z., Hastings J., Rombeau J. L., Settle R. G. Parenteral nutrition supplemented with short-chain fatty acids: effect on the small-bowel mucosa in normal rats. Am J Clin Nutr. 1990 Apr;51(4):685–689. doi: 10.1093/ajcn/51.4.685. [DOI] [PubMed] [Google Scholar]
- Kripke S. A., De Paula J. A., Berman J. M., Fox A. D., Rombeau J. L., Settle R. G. Experimental short-bowel syndrome: effect of an elemental diet supplemented with short-chain triglycerides. Am J Clin Nutr. 1991 Apr;53(4):954–962. doi: 10.1093/ajcn/53.4.954. [DOI] [PubMed] [Google Scholar]
- Kripke S. A., Fox A. D., Berman J. M., Settle R. G., Rombeau J. L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989 Mar-Apr;13(2):109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Holst J., Håkanson R., Sundler F. Distribution and properties of glucagon immunoreactivity in the digestive tract of various mammals: an immunohistochemical and immunochemical study. Histochemistry. 1975 Sep 29;44(4):281–290. doi: 10.1007/BF00490364. [DOI] [PubMed] [Google Scholar]
- Messing B., Landais P., Goldfarb B., Irving M. Home parenteral nutrition in adults: a multicentre survey in Europe. Clin Nutr. 1989 Feb;8(1):3–9. doi: 10.1016/0261-5614(89)90018-6. [DOI] [PubMed] [Google Scholar]
- Mojsov S., Weir G. C., Habener J. F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987 Feb;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C. L., Ling V., Bourassa D. Small intestinal and colonic changes induced by a chemically defined diet. Dig Dis Sci. 1980 Feb;25(2):123–128. doi: 10.1007/BF01308310. [DOI] [PubMed] [Google Scholar]
- Nightingale J. M., Kamm M. A., van der Sijp J. R., Ghatei M. A., Bloom S. R., Lennard-Jones J. E. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the 'colonic brake' to gastric emptying. Gut. 1996 Aug;39(2):267–272. doi: 10.1136/gut.39.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale J. M., Kamm M. A., van der Sijp J. R., Morris G. P., Walker E. R., Mather S. J., Britton K. E., Lennard-Jones J. E. Disturbed gastric emptying in the short bowel syndrome. Evidence for a 'colonic brake'. Gut. 1993 Sep;34(9):1171–1176. doi: 10.1136/gut.34.9.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale J. M., Lennard-Jones J. E., Gertner D. J., Wood S. R., Bartram C. I. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992 Nov;33(11):1493–1497. doi: 10.1136/gut.33.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale J. M., Lennard-Jones J. E., Walker E. R., Farthing M. J. Jejunal efflux in short bowel syndrome. Lancet. 1990 Sep 29;336(8718):765–768. doi: 10.1016/0140-6736(90)93238-k. [DOI] [PubMed] [Google Scholar]
- Nordgaard I., Hansen B. S., Mortensen P. B. Colon as a digestive organ in patients with short bowel. Lancet. 1994 Feb 12;343(8894):373–376. doi: 10.1016/s0140-6736(94)91220-3. [DOI] [PubMed] [Google Scholar]
- Nordgaard I., Hansen B. S., Mortensen P. B. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am J Clin Nutr. 1996 Aug;64(2):222–231. doi: 10.1093/ajcn/64.2.222. [DOI] [PubMed] [Google Scholar]
- Nordgaard I., Mortensen P. B., Langkilde A. M. Small intestinal malabsorption and colonic fermentation of resistant starch and resistant peptides to short-chain fatty acids. Nutrition. 1995 Mar-Apr;11(2):129–137. [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Roberge J. N., Brubaker P. L. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993 Jul;133(1):233–240. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- Rocca A. S., Brubaker P. L. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999 Apr;140(4):1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- Rocca A. S., Brubaker P. L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology. 1995 Dec;136(12):5593–5599. doi: 10.1210/endo.136.12.7588313. [DOI] [PubMed] [Google Scholar]
- Rountree D. B., Ulshen M. H., Selub S., Fuller C. R., Bloom S. R., Ghatei M. A., Lund P. K. Nutrient-independent increases in proglucagon and ornithine decarboxylase messenger RNAs after jejunoileal resection. Gastroenterology. 1992 Aug;103(2):462–468. doi: 10.1016/0016-5085(92)90835-m. [DOI] [PubMed] [Google Scholar]
- Sagor G. R., Al-Mukhtar M. Y., Ghatei M. A., Wright N. A., Bloom S. R. The effect of altered luminal nutrition on cellular proliferation and plasma concentrations of enteroglucagon and gastrin after small bowel resection in the rat. Br J Surg. 1982 Jan;69(1):14–18. doi: 10.1002/bjs.1800690106. [DOI] [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Evidence for a humoral mechanism after small intestinal resection. Exclusion of gastrin but not enteroglucagon. Gastroenterology. 1983 May;84(5 Pt 1):902–906. [PubMed] [Google Scholar]
- Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation of isolated and denervated jejunal segment of the rat. Scand J Gastroenterol. 1989 Sep;24(7):886–890. doi: 10.3109/00365528909089230. [DOI] [PubMed] [Google Scholar]
- Savage A. P., Gornacz G. E., Adrian T. E., Ghatei M. A., Goodlad R. A., Wright N. A., Bloom S. R. Is raised plasma peptide YY after intestinal resection in the rat responsible for the trophic response? Gut. 1985 Dec;26(12):1353–1358. doi: 10.1136/gut.26.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield W. N. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39 (Suppl 1):5–41. [PubMed] [Google Scholar]
- Scott R. B., Kirk D., MacNaughton W. K., Meddings J. B. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998 Nov;275(5 Pt 1):G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- Stevens F. M., Flanagan R. W., O'Gorman D., Buchanan K. D. Glucagonoma syndrome demonstrating giant duodenal villi. Gut. 1984 Jul;25(7):784–791. doi: 10.1136/gut.25.7.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden K. A., Drozdowski L. A., Thomson A. B., McBurney M. I. Short-chain fatty acid-supplemented total parenteral nutrition alters intestinal structure, glucose transporter 2 (GLUT2) mRNA and protein, and proglucagon mRNA abundance in normal rats. Am J Clin Nutr. 1998 Jul;68(1):118–125. doi: 10.1093/ajcn/68.1.118. [DOI] [PubMed] [Google Scholar]
- Taylor R. G., Verity K., Fuller P. J. Ileal glucagon gene expression: ontogeny and response to massive small bowel resection. Gastroenterology. 1990 Sep;99(3):724–729. doi: 10.1016/0016-5085(90)90961-y. [DOI] [PubMed] [Google Scholar]
- Thim L., Moody A. J. The primary structure of porcine glicentin (proglucagon). Regul Pept. 1981 May;2(2):139–150. doi: 10.1016/0167-0115(81)90007-0. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Hill M., Asa S. L., Brubaker P. L., Drucker D. J. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997 Jul;273(1 Pt 1):E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- Tsai C. H., Hill M., Drucker D. J. Biological determinants of intestinotrophic properties of GLP-2 in vivo. Am J Physiol. 1997 Mar;272(3 Pt 1):G662–G668. doi: 10.1152/ajpgi.1997.272.3.G662. [DOI] [PubMed] [Google Scholar]
- Varndell I. M., Bishop A. E., Sikri K. L., Uttenthal L. O., Bloom S. R., Polak J. M. Localization of glucagon-like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. J Histochem Cytochem. 1985 Oct;33(10):1080–1086. doi: 10.1177/33.10.3900195. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978 Jan 10;253(1):69–76. [PubMed] [Google Scholar]
- Wøjdemann M., Wettergren A., Hartmann B., Hilsted L., Holst J. J. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab. 1999 Jul;84(7):2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- Wøjdemann M., Wettergren A., Hartmann B., Holst J. J. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998 Aug;33(8):828–832. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Boushey R. P., Drucker D. J., Brubaker P. L. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999 Jul;117(1):99–105. doi: 10.1016/s0016-5085(99)70555-x. [DOI] [PubMed] [Google Scholar]