Abstract
BACKGROUND/AIM—Proinflammatory cytokines are key factors in the pathogenesis of Crohn's disease (CD). Activation of nuclear factor kappa B (NFκB), which is involved in their gene transcription, is increased in the intestinal mucosa of CD patients. As butyrate enemas may be beneficial in treating colonic inflammation, we investigated if butyrate promotes this effect by acting on proinflammatory cytokine expression. METHODS—Intestinal biopsy specimens, isolated lamina propria cells (LPMC), and peripheral blood mononuclear cells (PBMC) were cultured with or without butyrate for assessment of secretion of tumour necrosis factor (TNF) and mRNA levels. NFκB p65 activation was determined by immunofluorescence and gene reporter experiments. Levels of NFκB inhibitory protein (IκBα) were analysed by western blotting. The in vivo efficacy of butyrate was assessed in rats with trinitrobenzene sulphonic acid (TNBS) induced colitis. RESULTS—Butyrate decreased TNF production and proinflammatory cytokine mRNA expression by intestinal biopsies and LPMC from CD patients. Butyrate abolished lipopolysaccharide (LPS) induced expression of cytokines by PBMC and transmigration of NFκB from the cytoplasm to the nucleus. LPS induced NFκB transcriptional activity was decreased by butyrate while IκBα levels were stable. Butyrate treatment also improved TNBS induced colitis. CONCLUSIONS—Butyrate decreases proinflammatory cytokine expression via inhibition of NFκB activation and IκBα degradation. These anti-inflammatory properties provide a rationale for assessing butyrate in the treatment of CD. Keywords: inflammation; butyrate; Crohn's disease; nuclear factor kappa B; cytokines
Full Text
The Full Text of this article is available as a PDF (242.1 KB).
Figure 1 .
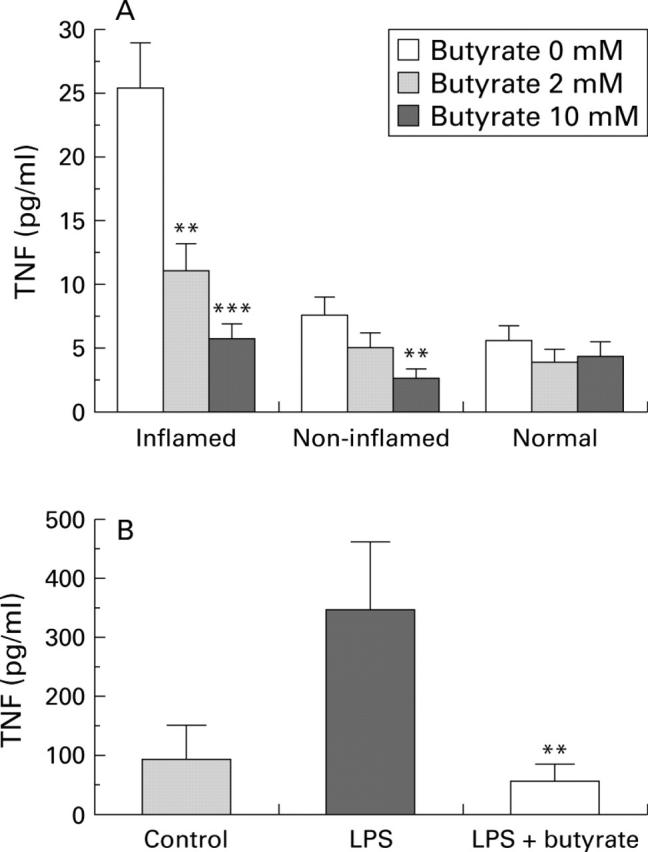
Effect of butyrate on tumour necrosis factor (TNF). Colonic biopsies (A) from inflamed (n=14) or non-inflamed (n=15) mucosa of patients with Crohn's disease or from normal (n=6) mucosa of healthy controls were cultured for 24 hours with or without 2 or 10 mM butyrate. **p<0.01,***p<0.001 v 0 mM. (B) Peripheral blood mononuclear cells (PBMC) (n=7) were cultured for 24 hours alone or in the presence of 2.5 µg/ml lipopolysaccharide (LPS) followed by 20 hours under the same conditions with or without 2 mM butyrate. Supernatant concentrations of total TNF were assessed by WEHI cell bioassay, and results are expressed as mean (SEM). **p<0.01 v LPS.
Figure 2 .
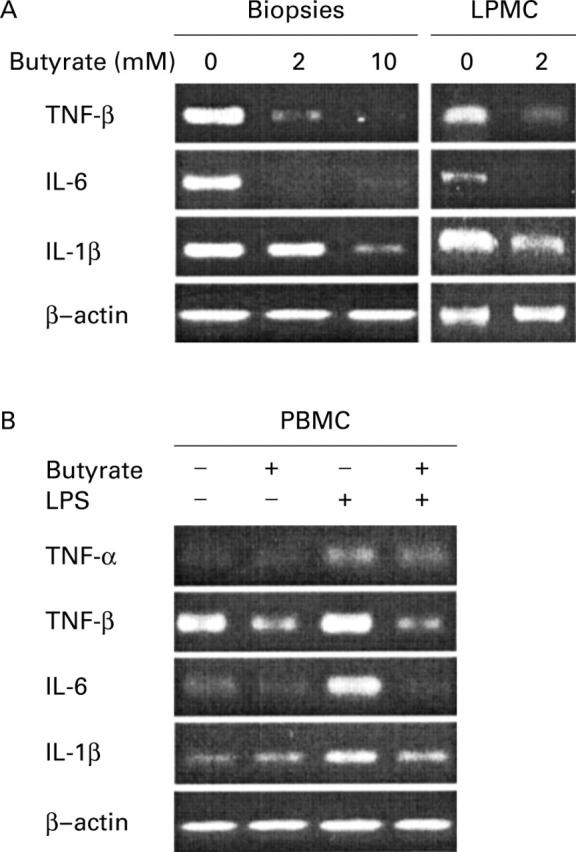
Effect of butyrate on proinflammatory cytokine mRNA. Biopsies and isolated lamina propria mononuclear cells (LPMC) (A) from inflamed mucosa of patients with Crohn's disease were cultured for 24 hours with or without 2 or 10 mM butyrate. Peripheral blood mononuclear cells (PBMC) (B) were cultured for 24 hours alone or in the presence of 2.5 µg/ml lipopolysaccharide (LPS) and then for another 20 hour period under the same conditions with or without 2 mM butyrate. RNA was extracted and RT-PCR was performed using primers designed for interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-β, TNF-α, and control β-actin.
Figure 3 .
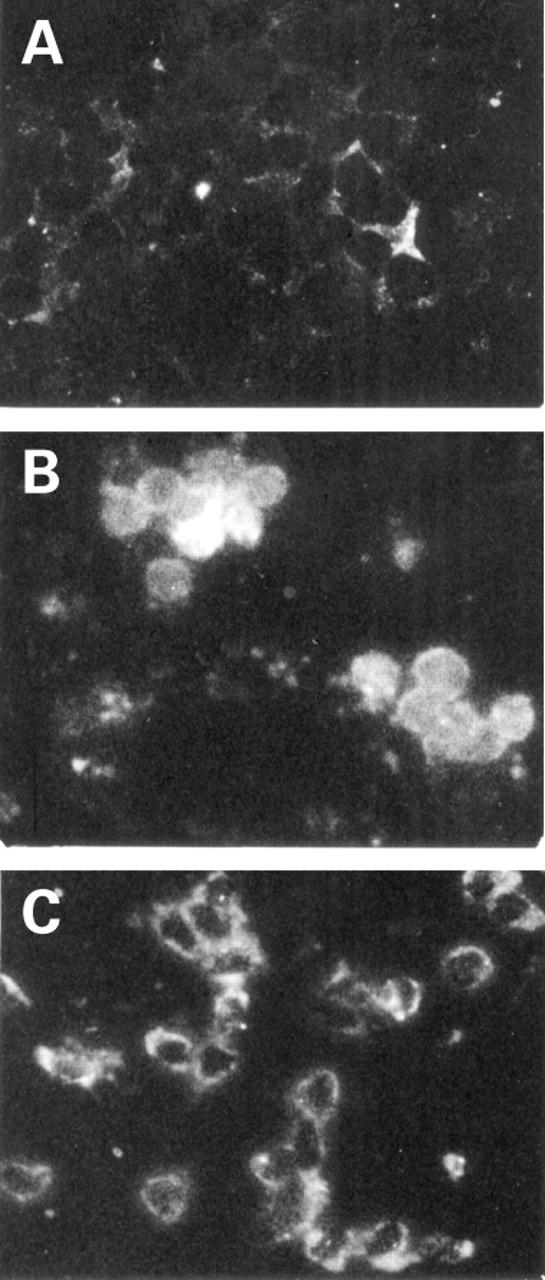
Effect of butyrate on nuclear translocation of nuclear factor kappa B (NFκB). Peripheral blood mononuclear cells (PBMC) (A) unstimulated, (B) lipopolysaccharide (LPS) stimulated, or (C) LPS stimulated and treated with 2 mM butyrate. The intracellular location of NFκB p65 (RelA) was determined by immunofluorescence using an anti-NFκB p65 polyclonal antibody. Magnification, ×400.
Figure 4 .
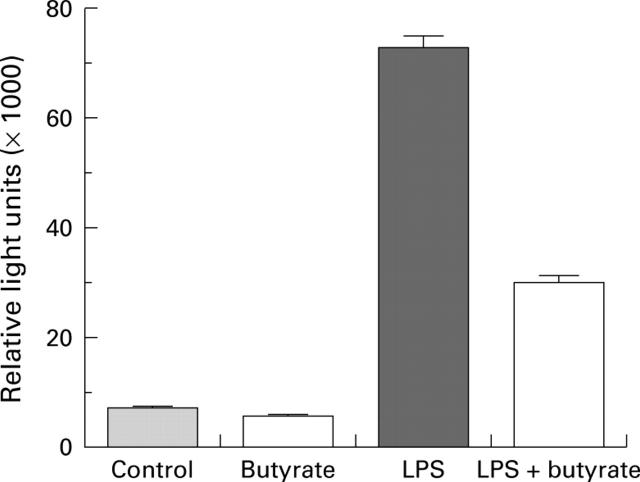
Effect of butyrate on nuclear factor kappa B (NFκB) transcriptional activity. THP-1 cells were transiently transfected with the NFκB reporter plasmid 3XMHC-luc and cultured alone or in the presence of 2.5 µg/ml lipopolysaccharide (LPS), with or without 2 mM butyrate for four hours. Luciferase activity was then determined. Data are mean (SEM) of four experiments.
Figure 5 .
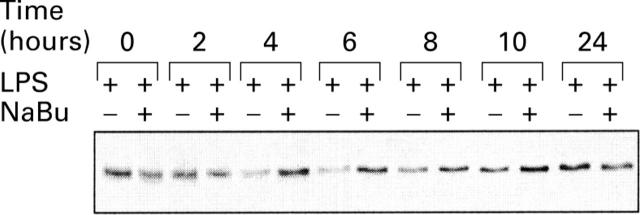
Effect of butyrate on inhibitor protein kappa Bα (IκBα) expression. THP-1 cells were stimulated for various periods of time with 2.5 µg/ml lipopolysaccharide (LPS), in the presence or absence of 2 mM butyrate. Cell lysates were subjected to western blot analysis of IκBα.
Figure 6 .
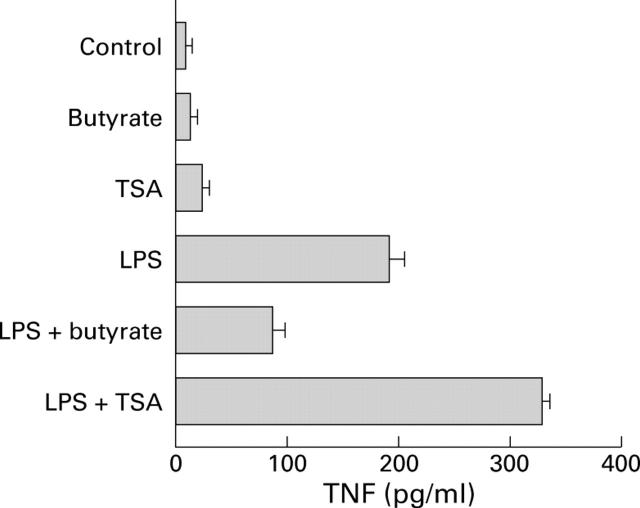
Comparison of the effects of butyrate and trichostatin A (TSA) on production of tumour necrosis factor (TNF). Peripheral blood mononuclear cells were cultured for 24 hours alone or in the presence of 2.5 µg/ml lipopolysaccharide (LPS) followed by 20 hours under the same conditions with or without 2 mM butyrate or 0.5 µM/ml of the histone deacetylase inhibitor TSA. Supernatant concentrations of total TNF were assessed by WEHI cell bioassay, and the results are expressed as mean (SEM) of three experiments.
Figure 7 .
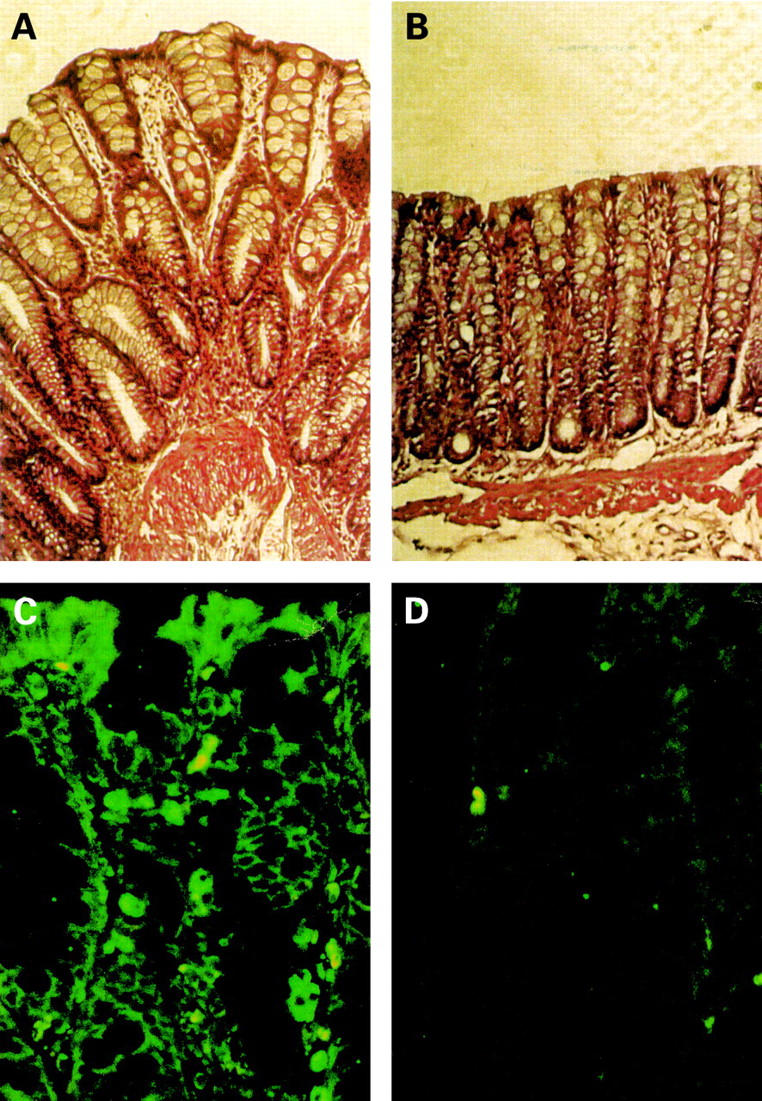
Effects of butyrate treatment on established colitis. Haematoxylin and eosin stained (A, B) paraffin sections of colon of rats with trinitrobenzene sulphonic acid (TNBS) induced colitis receiving isotonic saline (A) or 100 mM butyrate (B) enemas for two weeks. Magnification, ×200. Immunofluorescence staining (C, D) of paraffin sections of colon of rats with TNBS induced colitis receiving isotonic saline (C) or 100 mM butyrate (D) with a monoclonal antibody specific to the activated form of nuclear factor kappa B. Magnification, ×300.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh A., Fujiyama Y., Hata K., Araki Y., Takaya H., Shimada M., Bamba T. Counter-regulatory effect of sodium butyrate on tumour necrosis factor-alpha (TNF-alpha)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. Clin Exp Immunol. 1999 Oct;118(1):23–29. doi: 10.1046/j.1365-2249.1999.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auphan N., DiDonato J. A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baldassano R. N., Schreiber S., Johnston R. B., Jr, Fu R. D., Muraki T., MacDermott R. P. Crohn's disease monocytes are primed for accentuated release of toxic oxygen metabolites. Gastroenterology. 1993 Jul;105(1):60–66. doi: 10.1016/0016-5085(93)90010-a. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Barbier M., Cherbut C., Aubé A. C., Blottière H. M., Galmiche J. P. Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut. 1998 Dec;43(6):783–790. doi: 10.1136/gut.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G., Oudkerk Pool M., Scharenberg J. G., Kolkman J. J., von Blomberg B. M., Scheper R. J., Meuwissen S. G., Peña A. S. Differences in the intrinsic capacity of peripheral blood mononuclear cells to produce tumor necrosis factor alpha and beta in patients with inflammatory bowel disease and healthy controls. Scand J Gastroenterol. 1995 Nov;30(11):1095–1100. doi: 10.3109/00365529509101613. [DOI] [PubMed] [Google Scholar]
- Braegger C. P., Nicholls S., Murch S. H., Stephens S., MacDonald T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992 Jan 11;339(8785):89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Breese E. J., Michie C. A., Nicholls S. W., Murch S. H., Williams C. B., Domizio P., Walker-Smith J. A., MacDonald T. T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994 Jun;106(6):1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1996 Dec;8(6):872–877. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- Brynskov J., Nielsen O. H., Ahnfelt-Rønne I., Bendtzen K. Cytokines (immunoinflammatory hormones) and their natural regulation in inflammatory bowel disease (Crohn's disease and ulcerative colitis): a review. Dig Dis. 1994 Sep-Oct;12(5):290–304. doi: 10.1159/000171464. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzner J. D., Parmar R., Bell C. J., Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996 Apr;38(4):568–573. doi: 10.1136/gut.38.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmig G. A., Krieger P. M., Säemann M. D., Wenhardt C., Pohanka E., Zlabinger G. J. n-butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997 Oct;92(2):234–243. doi: 10.1046/j.1365-2567.1997.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammà C., Giunta M., Rosselli M., Cottone M. Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997 Nov;113(5):1465–1473. doi: 10.1053/gast.1997.v113.pm9352848. [DOI] [PubMed] [Google Scholar]
- Den Hond E., Hiele M., Evenepoel P., Peeters M., Ghoos Y., Rutgeerts P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology. 1998 Sep;115(3):584–590. doi: 10.1016/s0016-5085(98)70137-4. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gilbert K. M., Weigle W. O. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993 Aug 1;151(3):1245–1254. [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Mackay F., Browning J. L., Lawton P., Shah S. A., Comiskey M., Bhan A. K., Mizoguchi E., Terhorst C., Simpson S. J. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998 Dec;115(6):1464–1475. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- Maestri N. E., Brusilow S. W., Clissold D. B., Bassett S. S. Long-term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med. 1996 Sep 19;335(12):855–859. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]
- May M. J., Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998 Feb;19(2):80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991 Aug;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996 Sep;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Hiwatashi N., Liu Z., Toyota T. Secretion imbalance between tumour necrosis factor and its inhibitor in inflammatory bowel disease. Gut. 1998 Aug;43(2):203–209. doi: 10.1136/gut.43.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Lee J., Fusunyan R. D., MacDermott R. P., Sanderson I. R. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S. P., Ginder G. D., Faller D. V., Dover G. H., Ikuta T., Witkowska H. E., Cai S. P., Vichinsky E. P., Olivieri N. F. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993 Jan 14;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Plevy S. E., Landers C. J., Prehn J., Carramanzana N. M., Deem R. L., Shealy D., Targan S. R. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997 Dec 15;159(12):6276–6282. [PubMed] [Google Scholar]
- Reimund J. M., Wittersheim C., Dumont S., Muller C. D., Kenney J. S., Baumann R., Poindron P., Duclos B. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996 Nov;39(5):684–689. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980 Oct 4;2(8197):712–715. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P. A., Schölmerich J., Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998 Aug;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Rosales C., Juliano R. Integrin signaling to NF-kappa B in monocytic leukemia cells is blocked by activated oncogenes. Cancer Res. 1996 May 15;56(10):2302–2305. [PubMed] [Google Scholar]
- Ruddle N. H. Tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta). Curr Opin Immunol. 1992 Jun;4(3):327–332. doi: 10.1016/0952-7915(92)90084-r. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997 Dec;92(12 Suppl):5S–11S. [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994 Jan;35(1 Suppl):S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998 Apr;42(4):477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J., Hämling J., Koop I., Groessner B., Lochs H., Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999 Feb 6;353(9151):459–461. doi: 10.1016/S0140-6736(98)03339-X. [DOI] [PubMed] [Google Scholar]
- Siavoshian S., Blottière H. M., Bentouimou N., Cherbut C., Galmiche J. P. Butyrate enhances major histocompatibility complex class I, HLA-DR and ICAM-1 antigen expression on differentiated human intestinal epithelial cells. Eur J Clin Invest. 1996 Sep;26(9):803–810. doi: 10.1046/j.1365-2362.1996.2180561.x. [DOI] [PubMed] [Google Scholar]
- Stack W. A., Mann S. D., Roy A. J., Heath P., Sopwith M., Freeman J., Holmes G., Long R., Forbes A., Kamm M. A. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease. Lancet. 1997 Feb 22;349(9051):521–524. doi: 10.1016/s0140-6736(97)80083-9. [DOI] [PubMed] [Google Scholar]
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Wu G. D., Huang N., Wen X., Keilbaugh S. A., Yang H. High-level expression of I kappa B-beta in the surface epithelium of the colon: in vitro evidence for an immunomodulatory role. J Leukoc Biol. 1999 Dec;66(6):1049–1056. doi: 10.1002/jlb.66.6.1049. [DOI] [PubMed] [Google Scholar]