Abstract
BACKGROUND—Changes in substance P content and a relationship between the degree of perineural inflammation and pain has been demonstrated in chronic pancreatitis. Whether a relationship exists between neural alteration and pancreatic inflammation (neurogenic inflammation) is not known. AIMS—In the present study we evaluated gene expression of preprotachykinin A (PPT-A), the gene encoding substance P, and interleukin 8, a proinflammatory and hyperalgesic mediator whose release is co-regulated by substance P. PATIENTS—Pancreatic tissue specimens obtained from 21 patients (16 male, five female) with chronic pancreatitis and 18 healthy organ donors (nine male, nine female) were analysed. METHODS—Gene expression of PPT-A and interleukin 8 was studied by northern blot analysis. Respective proteins were localised using immunohistochemistry. RESULTS—Northern blot analysis showed that PTT-A mRNA expression levels were present at comparable levels in normal and chronic pancreatitis tissue samples. In contrast, interleukin 8 mRNA was expressed at very low levels in normal controls but was increased 41-fold (p<0.001) in chronic pancreatitis tissue samples. Using immunohistochemistry, interleukin 8 protein was localised mainly in immune cells often found around enlarged pancreatic nerves. In addition, in chronic pancreatitis, intense interleukin 8 immunostaining was present in metaplastic ductal cells of the atrophic pancreatic parenchyma. In chronic pancreatitis samples there was a positive relationship between interleukin 8 mRNA levels and the presence of ductal metaplasia (r=0.795; p<0.001) and the inflammation score (r=0.713; p<0.001). CONCLUSIONS—Our data indicate that in chronic pancreatitis, the increase in substance P in enlarged pancreatic nerves is not caused by enhanced intrapancreatic PTT-A mRNA expression, suggesting that the location of substance P synthesis is outside of the pancreas. In addition, localisation of interleukin 8 positive immune cells around pancreatic nerves further supports the existence of neuroimmune interactions as a pathophysiological mechanism in chronic pancreatitis. Keywords: chronic pancreatitis; interleukin 8; neurogenic inflammation; preprotachykinin A; substance P; immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (198.2 KB).
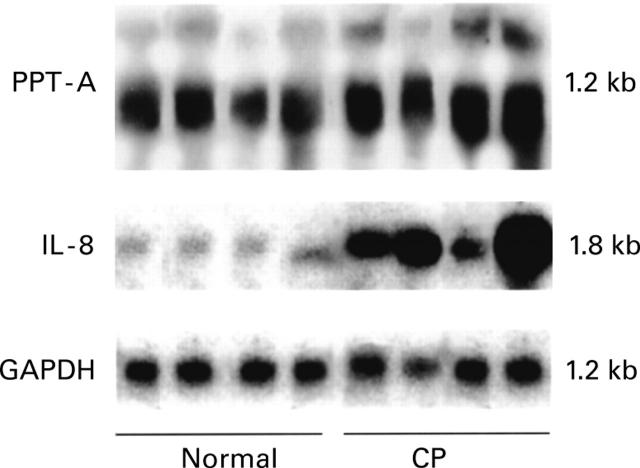
Figure 1 .
Northern blot analysis. Preprotachykinin A (PPT-A) and interleukin 8 (IL-8) mRNA expression in the normal pancreas (first four lanes) and in tissue samples from patients with chronic pancreatitis (CP) (latter four lanes). PPT-A mRNA was detectable in comparable levels in normal and CP tissue samples. In contrast, enhanced levels of IL-8 mRNA were present in CP samples. The filters were rehybridised with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA to verify equivalent RNA loading and RNA transfer. PPT-A mRNA migrates as a 1.2 kb band and IL-8 as a 1.8 kb band.
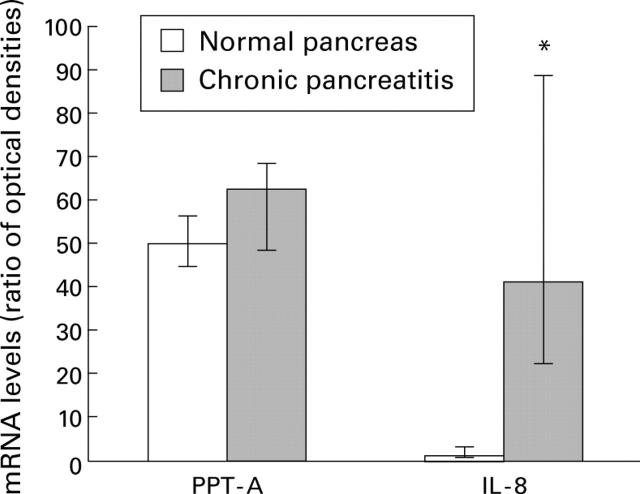
Figure 2 .
Densitometric analysis of northern blots. Preprotachykinin A (PPT-A), interleukin 8 (IL-8), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were analysed by laser densitometry in the normal pancreas and in chronic pancreatitis. The ratio of the optical density between PPT-A, IL-8, and the corresponding GAPDH signal was calculated. Bars represent the median with upper and lower quartiles. *p<0.001.
Figure 3 .
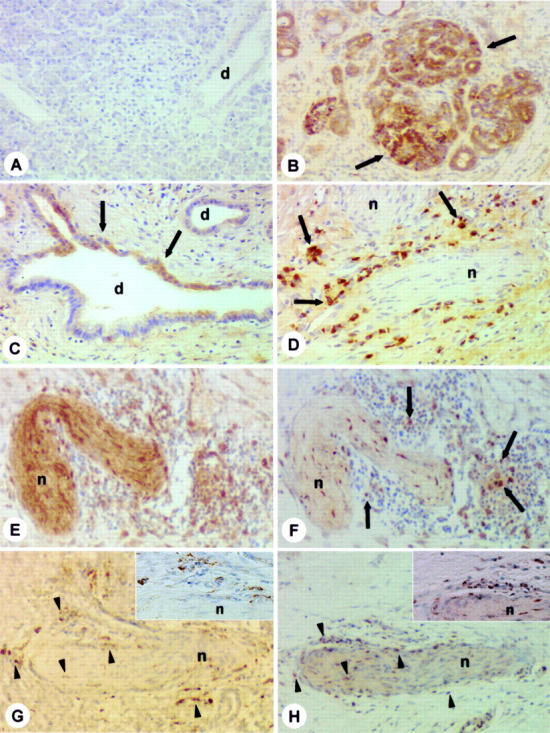
Immunohistochemical analysis. Interleukin 8 (IL-8) immunohistochemical analysis in the normal pancreas (A) and in chronic pancreatitis (CP) (B-D, F, H). In the normal pancreas (A), no IL-8 immunostaining was detectable. In contrast, in CP tissue sections (B-D, F, H), moderate to intense IL-8 immunoreactivity was found, mainly in the inflammatory cells (D, F, arrows) surrounding enlarged pancreatic nerves. In some cases those nerves were substance P immunoreactive (E). Panels (G) and (H) (arrowheads) show staining of consecutive tissue sections of CP for CD-68 (G) and IL-8 (H). The inserts in (G) and (H) show that IL-8 immunoreactive cells (H) are most probably also stained CD-68 positive (G). In addition, in CP tissue samples, moderate to strong IL-8 immunoreactivity was also found in metaplastic ductal cells (B, C, arrows). n=nerve; d=duct. (A) IL-8 immunostaining; (B- D, F, H) IL-8 immunostaining (patients Nos 14, 16, see table 1); (E) substance P immunostaining (patient No 14); (G) CD-68 immunostaining (patient No 16). Original magnification ×200.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Lloyd A. R. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997 Feb 15;349(9050):490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson J. M., Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987 Jan;67(1):67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Bockman D. E., Buchler M., Malfertheiner P., Beger H. G. Analysis of nerves in chronic pancreatitis. Gastroenterology. 1988 Jun;94(6):1459–1469. doi: 10.1016/0016-5085(88)90687-7. [DOI] [PubMed] [Google Scholar]
- Büchler M., Weihe E., Friess H., Malfertheiner P., Bockman E., Müller S., Nohr D., Beger H. G. Changes in peptidergic innervation in chronic pancreatitis. Pancreas. 1992;7(2):183–192. doi: 10.1097/00006676-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano P., Fink T., Weihe E., Friess H., Innocenti P., Beger H. G., Büchler M. W. Immune cell infiltration and growth-associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology. 1997 May;112(5):1648–1655. doi: 10.1016/s0016-5085(97)70047-7. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano P., Fink T., di Mola F. F., Weihe E., Innocenti P., Friess H., Büchler M. W. Neuroimmune appendicitis. Lancet. 1999 Aug 7;354(9177):461–466. doi: 10.1016/S0140-6736(98)10463-4. [DOI] [PubMed] [Google Scholar]
- Fink T., Di Sebastiano P., Büchler M., Beger H. G., Weihe E. Growth-associated protein-43 and protein gene-product 9.5 innervation in human pancreas: changes in chronic pancreatitis. Neuroscience. 1994 Nov;63(1):249–266. doi: 10.1016/0306-4522(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Friess H., Cantero D., Graber H., Tang W. H., Guo X., Kashiwagi M., Zimmermann A., Gold L., Korc M., Büchler M. W. Enhanced urokinase plasminogen activation in chronic pancreatitis suggests a role in its pathogenesis. Gastroenterology. 1997 Sep;113(3):904–913. doi: 10.1016/s0016-5085(97)70186-0. [DOI] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Beger H. G., Do D. A., Kobrin M. S., Korc M. Increased expression of acidic and basic fibroblast growth factors in chronic pancreatitis. Am J Pathol. 1994 Jan;144(1):117–128. [PMC free article] [PubMed] [Google Scholar]
- Gates T. S., Zimmerman R. P., Mantyh C. R., Vigna S. R., Mantyh P. W. Calcitonin gene-related peptide-alpha receptor binding sites in the gastrointestinal tract. Neuroscience. 1989;31(3):757–770. doi: 10.1016/0306-4522(89)90439-9. [DOI] [PubMed] [Google Scholar]
- Goldin E., Karmeli F., Selinger Z., Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci. 1989 May;34(5):754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Fisher M. C. Potentiation of IL-1-induced BALB/3T3 fibroblast proliferation by neuropeptides. J Immunol. 1988 Dec 15;141(12):4203–4208. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Mallet-Guy P. A. Late and very late results of resections of the nervous system in the treatment of chronic relapsing pancreatitis. Am J Surg. 1983 Feb;145(2):234–238. doi: 10.1016/0002-9610(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Mantyh C. R., Gates T. S., Zimmerman R. P., Welton M. L., Passaro E. P., Jr, Vigna S. R., Maggio J. E., Kruger L., Mantyh P. W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988 May;85(9):3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Catton M. D., Boehmer C. G., Welton M. L., Passaro E. P., Jr, Maggio J. E., Vigna S. R. Receptors for sensory neuropeptides in human inflammatory diseases: implications for the effector role of sensory neurons. Peptides. 1989 May-Jun;10(3):627–645. doi: 10.1016/0196-9781(89)90154-x. [DOI] [PubMed] [Google Scholar]
- Marchand J. E., Zaccheo T. S., Connelly C. S., Kream R. M. Selective in situ hybridization histochemical analyses of alternatively spliced mRNAs encoding beta- and gamma-preprotachykinins in rat central nervous system. Brain Res Mol Brain Res. 1993 Jan;17(1-2):83–94. doi: 10.1016/0169-328x(93)90076-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Christophers E. Purification and partial biologic characterization of a human lymphocyte-derived peptide with potent neutrophil-stimulating activity. J Immunol. 1988 May 15;140(10):3534–3540. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Serra M. C., Calzetti F., Ceska M., Cassatella M. A. Effect of substance P on superoxide anion and IL-8 production by human PMNL. Immunology. 1994 May;82(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Taub D. D., Oppenheim J. J. Chemokines, inflammation and the immune system. Ther Immunol. 1994 Aug;1(4):229–246. [PubMed] [Google Scholar]
- di Mola F. F., Friess H., Martignoni M. E., Di Sebastiano P., Zimmermann A., Innocenti P., Graber H., Gold L. I., Korc M., Büchler M. W. Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg. 1999 Jul;230(1):63–71. doi: 10.1097/00000658-199907000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mola F. F., Friess H., Scheuren A., Di Sebastiano P., Graber H., Egger B., Zimmermann A., Korc M., Büchler M. W. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn's disease. Ann Surg. 1999 Jan;229(1):67–75. doi: 10.1097/00000658-199901000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]