Abstract
BACKGROUND—Perinuclear antineutrophil cytoplasmic antibodies (pANCA) have been detected in a clinically distinct Crohn's disease subpopulation. Antibodies to Saccharomyces cerevisiae (ASCA) have been demonstrated in the majority of patients with Crohn's disease. AIMS—To examine the relationship between selective marker antibody expression in Crohn's disease and disease onset, location, and clinical behaviour patterns. METHODS—Sera from 156 consecutive patients with established Crohn's disease were evaluated in a blinded fashion for the presence of ASCA and ANCA. Clinical profiles were generated by investigators blinded to immune marker status. RESULTS—Using multiple regression analyses, higher ASCA levels were shown to be independently associated with early age of disease onset as well as both fibrostenosing and internal penetrating disease behaviours. Higher ANCA levels were associated with later age of onset and ulcerative colitis-like behaviour. Substratification of the Crohn's disease population using selective ANCA and ASCA expression (high levels of a single marker antibody): (1) distinguished homogeneous subgroups that manifested similar disease location and behaviours; and (2) identified patients with more aggressive small bowel disease. CONCLUSIONS—The findings suggest that by taking into account the magnitude of the host immune response, Crohn's disease can now be stratified on an immunological basis into more homogeneous clinically distinct subgroups, characterised by greater uniformity among anatomical distribution of disease and disease behaviour. Keywords: antineutrophil cytoplasmic antibody; anti-Saccharomyces cerevisiae antibody; Crohn's disease; inflammatory bowel disease; ulcerative colitis
Full Text
The Full Text of this article is available as a PDF (199.5 KB).
Figure 1 .
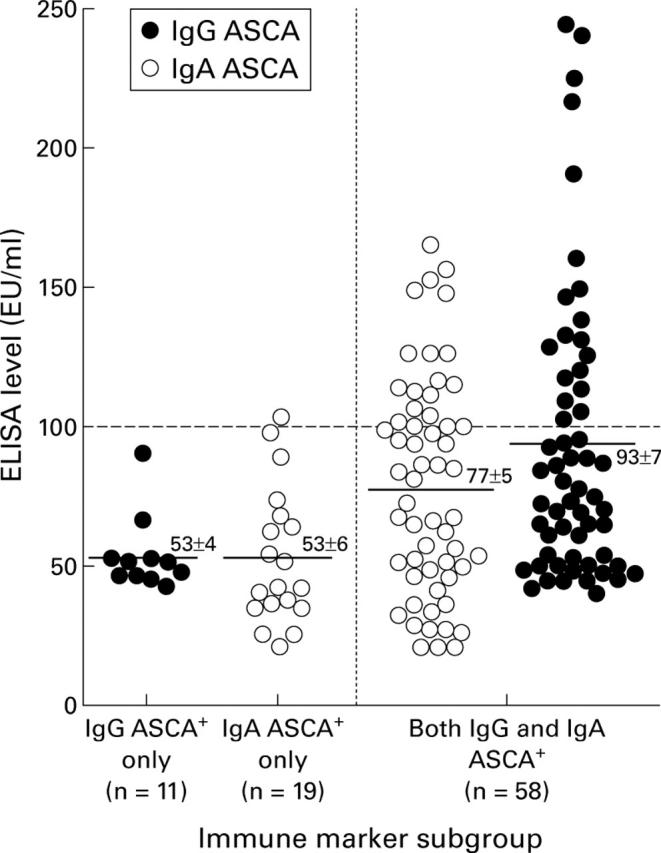
Levels of anti-Saccharomyces cerevisiae antibody (ASCA) positive sera from the Crohn's disease (CD) population. The solid line in each group represents the mean of the subgroup. Almost all patients (25/26) expressing the highest levels of either IgG or IgA ASCA (⩾100 EU/ml, values above the broken line) were part of the CD subgroup expressing both IgG and IgA ASCA.
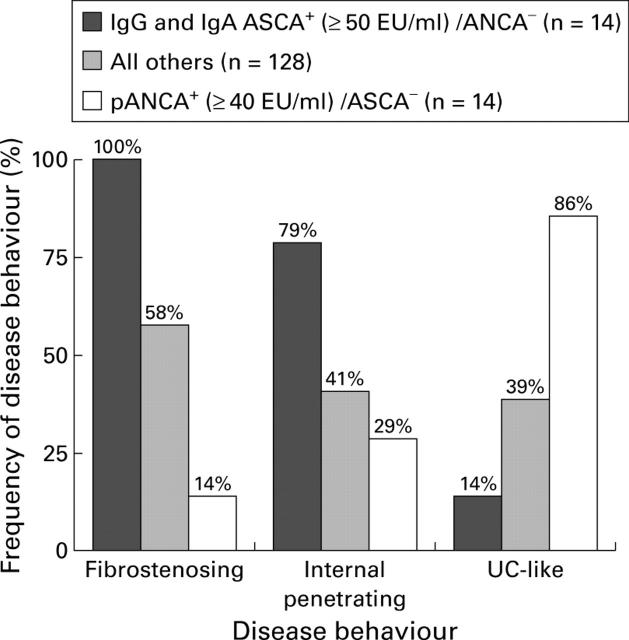
Figure 2 .
Substratification using selected expression of immune markers. Disease behaviour characteristics were examined in more immunologically homogeneous subgroups of patients with Crohn's disease (CD) (those expressing high levels of a single marker antibody) and compared with all other CD study patients. Overall differences in proportions were evaluated using the χ2 test for trend (p<0.001 for each of the disease behaviour characteristics).
Figure 3 .
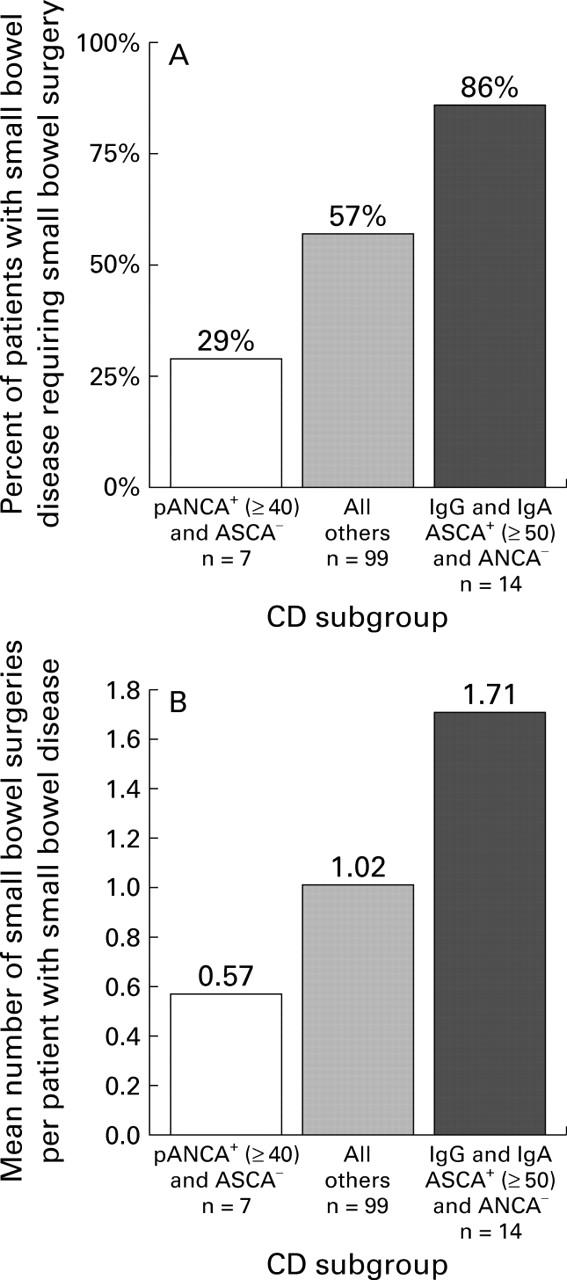
Surgery as a measure of disease aggressiveness. Substratification of the subset of patients with small bowel disease (Crohn's disease (CD) involving the small bowel alone or in combination with the colon) using selective immune marker expression. (A) Percentage of patients requiring small bowel surgery and (B) total number of small bowel surgeries per patient with small bowel involvement. Overall differences in proportions were evaluated using the χ2 test for trend. (A: p<0.0001; B: p<0.005).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard P., Berchtold W., Riedtmann H. J., Stadelmann G. Surgical recurrence of perforating and nonperforating Crohn's disease. A study of 101 surgically treated Patients. Dis Colon Rectum. 1996 Jan;39(1):80–87. doi: 10.1007/BF02048274. [DOI] [PubMed] [Google Scholar]
- Barclay G. R., McKenzie H., Pennington J., Parratt D., Pennington C. R. The effect of dietary yeast on the activity of stable chronic Crohn's disease. Scand J Gastroenterol. 1992;27(3):196–200. doi: 10.3109/00365529208999948. [DOI] [PubMed] [Google Scholar]
- Bayless T. M., Tokayer A. Z., Polito J. M., 2nd, Quaskey S. A., Mellits E. D., Harris M. L. Crohn's disease: concordance for site and clinical type in affected family members--potential hereditary influences. Gastroenterology. 1996 Sep;111(3):573–579. doi: 10.1053/gast.1996.v111.pm8780559. [DOI] [PubMed] [Google Scholar]
- Bouma G., Poen A. C., García-González M. A., Schreuder G. M., Felt-Bersma R. J., Meuwissen S. G., Pena A. S. HLA-DRB1*03, but not the TNFA -308 promoter gene polymorphism, confers protection against fistulising Crohn's disease. Immunogenetics. 1998 May;47(6):451–455. doi: 10.1007/s002510050382. [DOI] [PubMed] [Google Scholar]
- Cole G. F., Stuart C. A. A long perspective on childhood multiple sclerosis. Dev Med Child Neurol. 1995 Aug;37(8):661–666. doi: 10.1111/j.1469-8749.1995.tb15010.x. [DOI] [PubMed] [Google Scholar]
- Cooke W. T. Long term prognosis of Crohn's disease with onset in childhood and adolescence. Gut. 1984 Nov;25(11):1303–1305. doi: 10.1136/gut.25.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darroch C. J., Christmas S. E., Barnes R. M. In vitro human lymphocyte proliferative responses to a glycoprotein of the yeast Saccharomyces cerevisiae. Immunology. 1994 Feb;81(2):247–252. [PMC free article] [PubMed] [Google Scholar]
- Devlin H. B., Datta D., Dellipiani A. W. The incidence and prevalence of inflammatory bowel disease in North Tees Health District. World J Surg. 1980;4(2):183–193. doi: 10.1007/BF02393573. [DOI] [PubMed] [Google Scholar]
- Duerr R. H., Targan S. R., Landers C. J., LaRusso N. F., Lindsay K. L., Wiesner R. H., Shanahan F. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991 May;100(5 Pt 1):1385–1391. [PubMed] [Google Scholar]
- Elmgreen J., Sørensen H., Berkowicz A. Polymorphism of complement C3 in chronic inflammatory bowel disease. Predominance of the C3F gene in Crohn's disease. Acta Med Scand. 1984;215(4):375–378. doi: 10.1111/j.0954-6820.1984.tb05021.x. [DOI] [PubMed] [Google Scholar]
- Ettenger R. B. Children are different: the challenges of pediatric renal transplantation. Am J Kidney Dis. 1992 Dec;20(6):668–672. doi: 10.1016/s0272-6386(12)70238-x. [DOI] [PubMed] [Google Scholar]
- Farmer R. G., Whelan G., Fazio V. W. Long-term follow-up of patients with Crohn's disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985 Jun;88(6):1818–1825. doi: 10.1016/0016-5085(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Fleischer D. E., Grimm I. S., Friedman L. S. Inflammatory bowel disease in older patients. Med Clin North Am. 1994 Nov;78(6):1303–1319. doi: 10.1016/s0025-7125(16)30102-x. [DOI] [PubMed] [Google Scholar]
- Giaffer M. H., Clark A., Holdsworth C. D. Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance. Gut. 1992 Aug;33(8):1071–1075. doi: 10.1136/gut.33.8.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein A. J., Lachman P., Sachar D. B., Springhorn J., Heimann T., Janowitz H. D., Aufses A. H., Jr Perforating and non-perforating indications for repeated operations in Crohn's disease: evidence for two clinical forms. Gut. 1988 May;29(5):588–592. doi: 10.1136/gut.29.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Saverymuttu S. H., Keshavarzian A., Hodgson H. J. Is the pattern of inflammatory bowel disease different in the elderly? Age Ageing. 1985 Nov;14(6):366–370. doi: 10.1093/ageing/14.6.366. [DOI] [PubMed] [Google Scholar]
- Halme L., von Smitten K., Husa A. The incidence of Crohn's disease in the Helsinki metropolitan area during 1975-1985. Ann Chir Gynaecol. 1989;78(2):115–119. [PubMed] [Google Scholar]
- Hesresbach D., Alizadeh M., Bretagne J. F., Gautier A., Quillivic F., Lemarchand B., Gosselin M., Genetet B., Semana G. Investigation of the association of major histocompatibility complex genes, including HLA class I, class II and TAP genes, with clinical forms of Crohn's disease. Eur J Immunogenet. 1996 Apr;23(2):141–151. doi: 10.1111/j.1744-313x.1996.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Høst A., Jacobsen H. P., Halken S., Holmenlund D. The natural history of cow's milk protein allergy/intolerance. Eur J Clin Nutr. 1995 Sep;49 (Suppl 1):S13–S18. [PubMed] [Google Scholar]
- Lenaerts C., Roy C. C., Vaillancourt M., Weber A. M., Morin C. L., Seidman E. High incidence of upper gastrointestinal tract involvement in children with Crohn disease. Pediatrics. 1989 May;83(5):777–781. [PubMed] [Google Scholar]
- Lindberg E., Magnusson K. E., Tysk C., Järnerot G. Antibody (IgG, IgA, and IgM) to baker's yeast (Saccharomyces cerevisiae), yeast mannan, gliadin, ovalbumin and betalactoglobulin in monozygotic twins with inflammatory bowel disease. Gut. 1992 Jul;33(7):909–913. doi: 10.1136/gut.33.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashako M. N., Cezard J. P., Navarro J., Mougenot J. F., Sonsino E., Gargouri A., Maherzi A. Crohn's disease lesions in the upper gastrointestinal tract: correlation between clinical, radiological, endoscopic, and histological features in adolescents and children. J Pediatr Gastroenterol Nutr. 1989 May;8(4):442–446. [PubMed] [Google Scholar]
- McKenzie H., Main J., Pennington C. R., Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990 May;31(5):536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsén U., Bernell O., Johansson C., Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with Crohn's disease. Scand J Gastroenterol. 1991 Mar;26(3):302–306. doi: 10.3109/00365529109025046. [DOI] [PubMed] [Google Scholar]
- Norlén B. J., Krause U., Bergman L. An epidemiological study of Crohn's disease. Scand J Gastroenterol. 1970;5(5):385–390. [PubMed] [Google Scholar]
- O'Keefe E. A., Wright J. P., Froggatt J., Zabow D. Medium-term follow-up of Crohn's disease in Cape Town. S Afr Med J. 1989 Aug 19;76(4):139–141. [PubMed] [Google Scholar]
- Patel R. T., Stokes R., Birch D., Ibbotson J., Keighley M. R. Influence of total colectomy on serum antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Br J Surg. 1994 May;81(5):724–726. doi: 10.1002/bjs.1800810535. [DOI] [PubMed] [Google Scholar]
- Peeters M., Nevens H., Baert F., Hiele M., de Meyer A. M., Vlietinck R., Rutgeerts P. Familial aggregation in Crohn's disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology. 1996 Sep;111(3):597–603. doi: 10.1053/gast.1996.v111.pm8780562. [DOI] [PubMed] [Google Scholar]
- Polito J. M., 2nd, Childs B., Mellits E. D., Tokayer A. Z., Harris M. L., Bayless T. M. Crohn's disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996 Sep;111(3):580–586. doi: 10.1053/gast.1996.v111.pm8780560. [DOI] [PubMed] [Google Scholar]
- Polito J. M., 2nd, Rees R. C., Childs B., Mendeloff A. I., Harris M. L., Bayless T. M. Preliminary evidence for genetic anticipation in Crohn's disease. Lancet. 1996 Mar 23;347(9004):798–800. doi: 10.1016/s0140-6736(96)90870-3. [DOI] [PubMed] [Google Scholar]
- Puntis J., McNeish A. S., Allan R. N. Long term prognosis of Crohn's disease with onset in childhood and adolescence. Gut. 1984 Apr;25(4):329–336. doi: 10.1136/gut.25.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton J. F., Sendid B., Reumaux D., Duthilleul P., Cortot A., Grandbastien B., Charrier G., Targan S. R., Colombel J. F., Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998 Jun;42(6):788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruemmele F. M., Targan S. R., Levy G., Dubinsky M., Braun J., Seidman E. G. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998 Oct;115(4):822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Geboes K., Vantrappen G., Beyls J., Kerremans R., Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990 Oct;99(4):956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- Sachar D. B., Subramani K., Mauer K., Rivera-MacMurray S., Turtel P., Bodian C. A., Greenstein A. J. Patterns of postoperative recurrence in fistulizing and stenotic Crohn's disease. a retrospective cohort study of 71 patients. J Clin Gastroenterol. 1996 Mar;22(2):114–116. doi: 10.1097/00004836-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Sandborn W. J., Landers C. J., Tremaine W. J., Targan S. R. Antineutrophil cytoplasmic antibody correlates with chronic pouchitis after ileal pouch-anal anastomosis. Am J Gastroenterol. 1995 May;90(5):740–747. [PubMed] [Google Scholar]
- Sandborn W. J., Landers C. J., Tremaine W. J., Targan S. R. Association of antineutrophil cytoplasmic antibodies with resistance to treatment of left-sided ulcerative colitis: results of a pilot study. Mayo Clin Proc. 1996 May;71(5):431–436. doi: 10.4065/71.5.431. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Grootscholten C., Holt H., Jewell D. P. Clinical patterns of familial inflammatory bowel disease. Gut. 1996 May;38(5):738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J., Jewell D. P., Rosenberg W. M., Bell J. I. Genetics of inflammatory bowel disease. Gut. 1994 May;35(5):696–700. doi: 10.1136/gut.35.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J., Landers C. J., Welsh K. I., Koss K., Targan S., Jewell D. P. The presence of anti-neutrophil antibodies reflects clinical and genetic heterogeneity within inflammatory bowel disease. Inflamm Bowel Dis. 1998 Feb;4(1):18–26. doi: 10.1097/00054725-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Saxon A., Shanahan F., Landers C., Ganz T., Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990 Aug;86(2):202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- Sendid B., Colombel J. F., Jacquinot P. M., Faille C., Fruit J., Cortot A., Lucidarme D., Camus D., Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996 Mar;3(2):219–226. doi: 10.1128/cdli.3.2.219-226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan S. R., Landers C. J., Cobb L., MacDermott R. P., Vidrich A. Perinuclear anti-neutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells of ulcerative colitis patients. J Immunol. 1995 Sep 15;155(6):3262–3267. [PubMed] [Google Scholar]
- Targan S. R., Landers C., Vidrich A., Czaja A. J. High-titer antineutrophil cytoplasmic antibodies in type-1 autoimmune hepatitis. Gastroenterology. 1995 Apr;108(4):1159–1166. doi: 10.1016/0016-5085(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Wang S. J., Yang H. Y., Redford A., Magalong D., Tyan D., McElree C. K., Pressman S. R., Shanahan F., Targan S. R. Distinct associations of HLA class II genes with inflammatory bowel disease. Gastroenterology. 1993 Mar;104(3):741–748. doi: 10.1016/0016-5085(93)91009-7. [DOI] [PubMed] [Google Scholar]
- Tucker L. B., Menon S., Schaller J. G., Isenberg D. A. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995 Sep;34(9):866–872. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas E. A., Plevy S. E., Landers C. J., Binder S. W., Ferguson D. M., Yang H., Rotter J. I., Vidrich A., Targan S. R. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn's disease define a clinical subgroup. Gastroenterology. 1996 Jun;110(6):1810–1819. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- Vecchi M., Bianchi M. B., Calabresi C., Meucci G., Tatarella M., de Franchis R. Long-term observation of the perinuclear anti-neutrophil cytoplasmic antibody status in ulcerative colitis patients. Scand J Gastroenterol. 1998 Feb;33(2):170–173. doi: 10.1080/00365529850166905. [DOI] [PubMed] [Google Scholar]
- Vecchi M., Bianchi M. B., Sinico R. A., Radice A., Meucci G., Torgano G., Omodei P., Forzenigo L., Landoni M., Arrigoni M. Antibodies to neutrophil cytoplasm in Italian patients with ulcerative colitis: sensitivity, specificity and recognition of putative antigens. Digestion. 1994;55(1):34–39. doi: 10.1159/000201120. [DOI] [PubMed] [Google Scholar]
- Vecchi M., Gionchetti P., Bianchi M. B., Belluzzi A., Meucci G., Campieri M., de Franchis R. p-ANCA and development of pouchitis in ulcerative colitis patients after proctocolectomy and ileoanal pouch anastomosis. Lancet. 1994 Sep 24;344(8926):886–887. doi: 10.1016/s0140-6736(94)92859-2. [DOI] [PubMed] [Google Scholar]
- Vidrich A., Lee J., James E., Cobb L., Targan S. Segregation of pANCA antigenic recognition by DNase treatment of neutrophils: ulcerative colitis, type 1 autoimmune hepatitis, and primary sclerosing cholangitis. J Clin Immunol. 1995 Nov;15(6):293–299. doi: 10.1007/BF01541319. [DOI] [PubMed] [Google Scholar]
- Whelan G., Farmer R. G., Fazio V. W., Goormastic M. Recurrence after surgery in Crohn's disease. Relationship to location of disease (clinical pattern) and surgical indication. Gastroenterology. 1985 Jun;88(6):1826–1833. doi: 10.1016/0016-5085(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Yanase Y., Tango T., Okumura K., Tada T., Kawasaki T. Lymphocyte subsets identified by monoclonal antibodies in healthy children. Pediatr Res. 1986 Nov;20(11):1147–1151. doi: 10.1203/00006450-198611000-00017. [DOI] [PubMed] [Google Scholar]
- Yang H., McElree C., Roth M. P., Shanahan F., Targan S. R., Rotter J. I. Familial empirical risks for inflammatory bowel disease: differences between Jews and non-Jews. Gut. 1993 Apr;34(4):517–524. doi: 10.1136/gut.34.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Rotter J. I., Toyoda H., Landers C., Tyran D., McElree C. K., Targan S. R. Ulcerative colitis: a genetically heterogeneous disorder defined by genetic (HLA class II) and subclinical (antineutrophil cytoplasmic antibodies) markers. J Clin Invest. 1993 Aug;92(2):1080–1084. doi: 10.1172/JCI116613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E. Progression of allergy and asthma through childhood to adolescence. Thorax. 1996 Jan;51 (Suppl 1):S3–S6. doi: 10.1136/thx.51.suppl_1.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]