Abstract
BACKGROUND—Acute and chronic use of non-steroidal anti-inflammatory drugs can increase intestinal permeability. Rofecoxib, which selectively inhibits cyclooxygenase 2 (COX-2), is a novel anti-inflammatory drug with the potential to produce minimal gastrointestinal toxic effects while retaining clinical efficacy. AIMS—To assess the potential for rofecoxib to affect the intestine adversely, in comparison with placebo and indomethacin. SUBJECTS—Thirty nine healthy subjects (aged 24-30 years). METHOD—We performed a four period crossover trial to assess intestinal permeability before and after seven days of treatment. Permeability was measured by the urinary ratio of chromium-51 labelled ethylene diamine tetraacetate (51CrEDTA)/L-rhamnose (five hour collection). RESULTS—Indomethacin 50 mg three times daily produced greater increases in intestinal permeability compared with placebo or rofecoxib (25 or 50 mg) (p⩽0.001); rofecoxib was not significantly different from placebo. Mean day 7 to baseline ratios (95% confidence intervals) for 51CrEDTA/L-rhamnose were 0.97 (0.82, 1.16), 0.80 (0.68, 0.95), 0.98 (0.82, 1.17), and 1.53 (1.27, 1.85) for placebo, rofecoxib 25 mg, rofecoxib 50 mg, and indomethacin groups, respectively. Rofecoxib was generally well tolerated. CONCLUSION—In this study, treatment for one week with indomethacin 50 mg three times daily significantly increased intestinal permeability compared with placebo, while treatment with rofecoxib 25 mg or 50 mg daily did not. The absence of a significant effect of rofecoxib on intestinal permeability at doses at least twice those recommended to treat osteoarthritis was consistent with other studies that have demonstrated little or no injury to the gastrointestinal mucosa associated with rofecoxib therapy. Keywords: rofecoxib; COX-2 inhibitor; indomethacin; non-steroidal anti-inflammatory drugs; intestinal permeability; osteoarthritis
Full Text
The Full Text of this article is available as a PDF (149.7 KB).
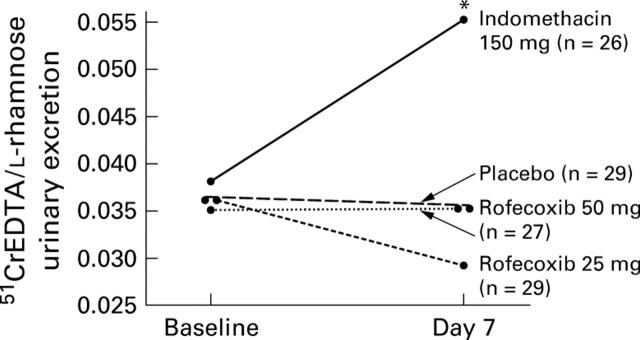
Figure 1 .
Five hour geometric mean urinary excretion ratios for 51CrEDTA/L-rhamnose at baseline and on day 7 for subjects who received placebo, rofecoxib 25 mg, rofecoxib 50 mg, and indomethacin 150 mg (per protocol analysis). Indomethacin 150 mg significantly increased the 51CrEDTA/L-rhamnose ratio compared with baseline values, whereas placebo, rofecoxib 25 mg, and rofecoxib 50 mg did not. *p<0.05 versus baseline values
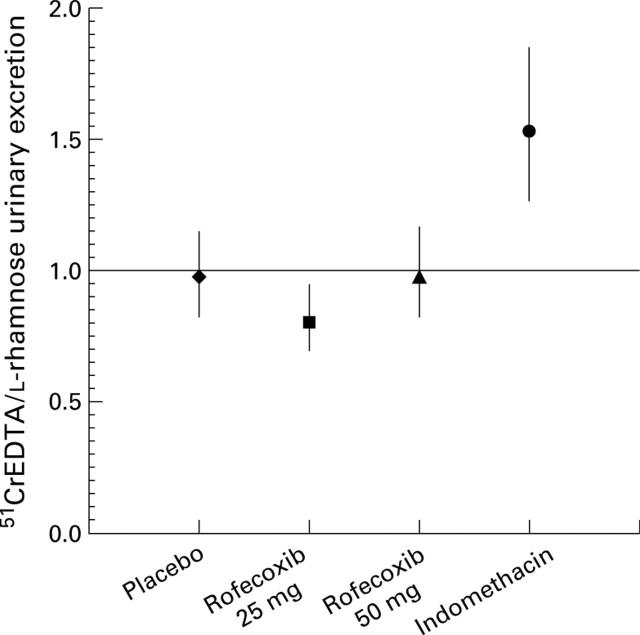
Figure 2 .
Day 7 to baseline geometric mean ratios (with 95% confidence intervals) for five hour urinary excretion of 51CrEDTA/L-rhamnose in subjects who received placebo, rofecoxib 25 mg, rofecoxib 50 mg, and indomethacin 150 mg (per protocol analysis).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., Fehilly B., Smethurst P., Menzies I. S., Levi A. J. Importance of local versus systemic effects of non-steroidal anti-inflammatory drugs in increasing small intestinal permeability in man. Gut. 1991 Mar;32(3):275–277. doi: 10.1136/gut.32.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., MacPherson A. J., Russell A. S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993 Jun;104(6):1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Maxton D., Reynolds A. P., Catt S., Peters T. J., Menzies I. S. Comparison of four markers of intestinal permeability in control subjects and patients with coeliac disease. Scand J Gastroenterol. 1994 Jul;29(7):630–639. doi: 10.3109/00365529409092484. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Price A. B., Zanelli G., Smethurst P., Burke M., Gumpel J. M., Levi A. J. Clinicopathological features of nonsteroidal antiinflammatory drug-induced small intestinal strictures. Gastroenterology. 1988 Apr;94(4):1070–1074. doi: 10.1016/0016-5085(88)90568-9. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Clark P., Menzies I., Levi J., Peters T. Effect of prostaglandin on indomethacin-induced increased intestinal permeability in man. Scand J Gastroenterol Suppl. 1989;164:97–103. doi: 10.3109/00365528909091195. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Fenn C. G., Lee C. E., Menzies I. S., Levi A. J. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1989 Mar;34(3):407–411. doi: 10.1007/BF01536263. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., Smethurst P., Peters T. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986 Nov;27(11):1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., So A., Zanelli G. D., Levi A. J., Gumpel J. M., Peters T. J., Ansell B. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet. 1984 Nov 24;2(8413):1171–1174. doi: 10.1016/s0140-6736(84)92739-9. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Prouse P., Smethurst P., Smith T., Levi S., Gumpel M. J., Levi A. J. Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet. 1987 Sep 26;2(8561):711–714. doi: 10.1016/s0140-6736(87)91075-0. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Smethurst P., Price A. B., Gumpel M. J., Levi A. J. The pathogenesis and consequence of non steroidal anti-inflammatory drug induced small intestinal inflammation in man. Scand J Rheumatol Suppl. 1987;64:55–62. doi: 10.3109/03009748709096722. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Boyce S., Brideau C., Charleson S., Cromlish W., Ethier D., Evans J., Ford-Hutchinson A. W., Forrest M. J., Gauthier J. Y. Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999 Aug;290(2):551–560. [PubMed] [Google Scholar]
- Dyer N. H., Kendall M. J., Hawkins C. F. Malabsorption in rheumatoid disease. Ann Rheum Dis. 1971 Nov;30(6):626–630. doi: 10.1136/ard.30.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E. W., Dallob A., De Lepeleire I., Van Hecken A., Riendeau D., Yuan W., Porras A., Wittreich J., Seibold J. R., De Schepper P. Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin Pharmacol Ther. 1999 Mar;65(3):336–347. doi: 10.1016/S0009-9236(99)70113-X. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci. 1998 Mar-Apr;28(2):67–81. [PubMed] [Google Scholar]
- Kendall M. J., Nutter S., Hawkins C. F. Xylose test: effect of aspirin and indomethacin. Br Med J. 1971 Mar 6;1(5748):533–536. doi: 10.1136/bmj.1.5748.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza F. L., Rack M. F., Simon T. J., Quan H., Bolognese J. A., Hoover M. E., Wilson F. R., Harper S. E. Specific inhibition of cyclooxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment Pharmacol Ther. 1999 Jun;13(6):761–767. doi: 10.1046/j.1365-2036.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Mahmud T., Rafi S. S., Scott D. L., Wrigglesworth J. M., Bjarnason I. Nonsteroidal antiinflammatory drugs and uncoupling of mitochondrial oxidative phosphorylation. Arthritis Rheum. 1996 Dec;39(12):1998–2003. doi: 10.1002/art.1780391208. [DOI] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Isenberg D. A., Hajirousou V., Tolfree S., Clark J., Snaith M. L. Preliminary evidence for gut involvement in the pathogenesis of rheumatoid arthritis? Br J Rheumatol. 1986 May;25(2):162–166. doi: 10.1093/rheumatology/25.2.162. [DOI] [PubMed] [Google Scholar]
- Sigthorsson G., Tibble J., Hayllar J., Menzies I., Macpherson A., Moots R., Scott D., Gumpel M. J., Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998 Oct;43(4):506–511. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S., Hayllar H., Rafi S., Wrigglesworth J. M., Macpherson A. J., Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gastroenterol. 1995 Apr;30(4):289–299. doi: 10.3109/00365529509093280. [DOI] [PubMed] [Google Scholar]
- Warner T. D., Giuliano F., Vojnovic I., Bukasa A., Mitchell J. A., Vane J. R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]