Abstract
BACKGROUND—When recombinant adenoviruses are infused directly into the circulation, transgene expression is almost completely restricted to the liver. AIMS—Efficiency and safety of adenovirus mediated gene transfer into damaged livers were examined in mice with liver cirrhosis or fulminant hepatitis. METHODS—Liver cirrhosis and fulminant hepatitis were induced by intraperitoneal administration of thioacetamide and D-galactosamine followed by lipopolysaccharide, respectively. Mice were infused with adenoviruses carrying the Escherichia coli β-galactosidase gene, lacZ gene, into the tail vein. Transduction efficiency of the lacZ gene was estimated histochemically by X-gal staining and quantitatively using a chemiluminescent assay. Activation of adenovirus specific T cells and development of neutralising antibodies against adenovirus were also examined. RESULTS—Histochemical evaluation revealed that approximately 40%, 80%, and 40% of cells in normal, cirrhotic, and fulminant hepatitis livers, respectively, were stained blue using X-gal staining. Quantitative analyses revealed that levels of lacZ expression in cirrhotic livers were approximately 2.5-fold and sixfold greater than those in normal and fulminant hepatitis livers, respectively. Although transgene expression in fulminant hepatitis livers was significantly lower than that in normal livers, marked levels of transgene expression were achieved even in fulminant hepatitis livers. Significant adverse effects of adenoviruses were not observed in damaged livers. There were no significant differences in cellular or humoral immune responses to adenoviruses among animals with normal, cirrhotic, and fulminant hepatitis livers. CONCLUSIONS—Our results suggest that gene therapy with adenoviruses may be used efficiently and safely, even in patients with severe liver disease. Keywords: adenovirus; transduction efficiency; safety; liver cirrhosis; fulminant hepatitis
Full Text
The Full Text of this article is available as a PDF (280.1 KB).
Figure 1 .
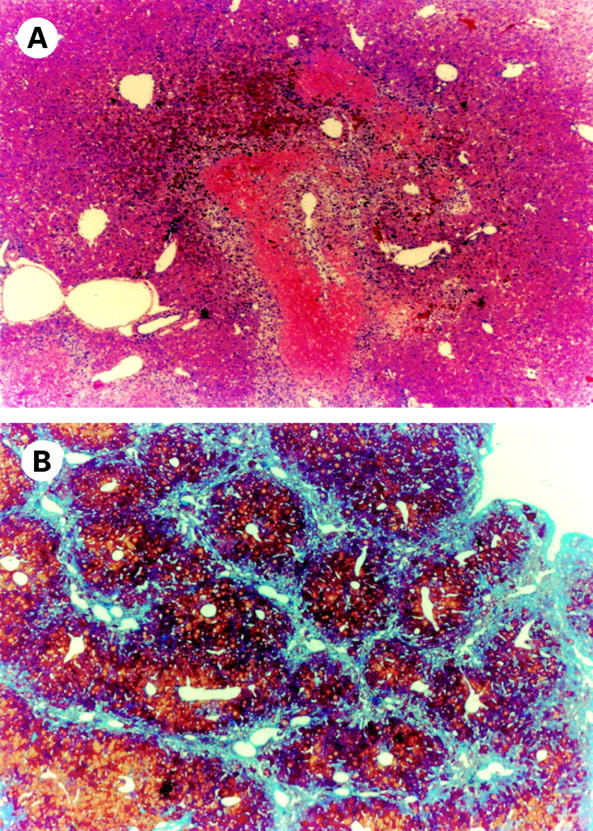
Histological analysis of fulminant hepatitis and liver cirrhosis models in mice. Haematoxylin-eosin staining of the liver of mice administered with D-galactosamine and lipopolysaccharide revealed acute severe hepatitis with massive haemorrhagic necrosis of hepatocytes, disarray of the lobules, and marked infiltration of inflammatory cells (A). Azan staining of the liver of mice treated with thioacetamide for 32 weeks revealed an increase and thickening of the fibrotic septa, traversing the lobular parenchyma, and formation of pseudolobuli (B). (Original magnification ×40.)
Figure 2 .
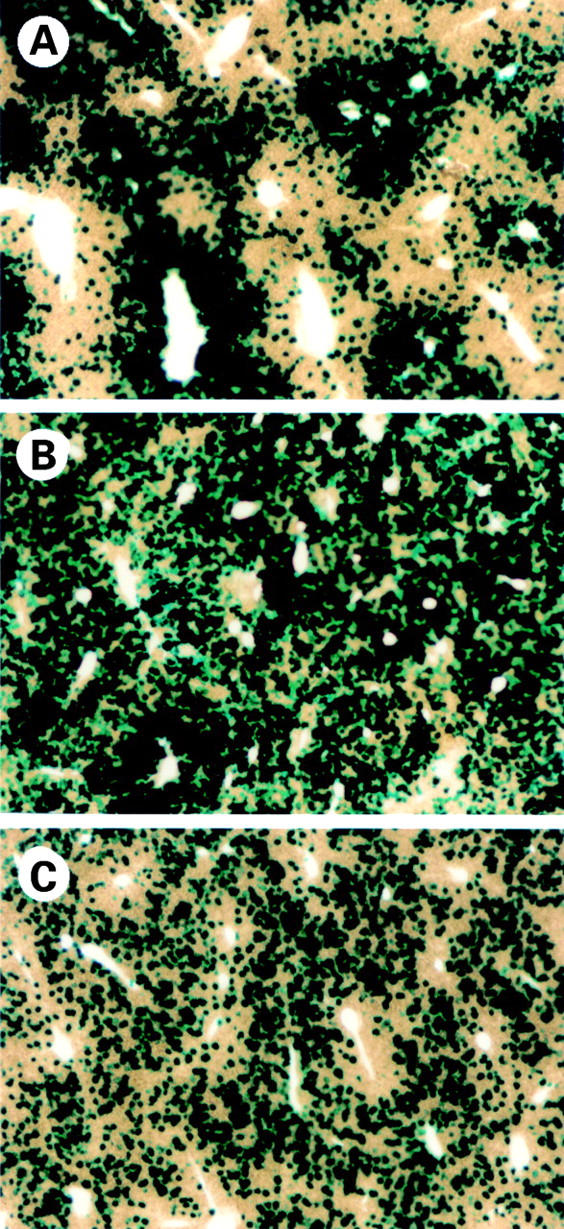
LacZ gene expression in the liver by intravenous administration of adenoviruses. Mice with normal, cirrhotic, and fulminant hepatitis livers were infused intravenously with adenoviruses (2×108 pfu/200 µl) carrying the lacZ gene. Animals were killed on day 4 after adenoviral infusion and lacZ gene expression was examined by X-gal staining. Approximately 40%, 80%, and 40% of the whole liver was stained blue in the normal (A), cirrhotic (B), and fulminant hepatitis (C) livers, respectively. (Original magnification ×40.)
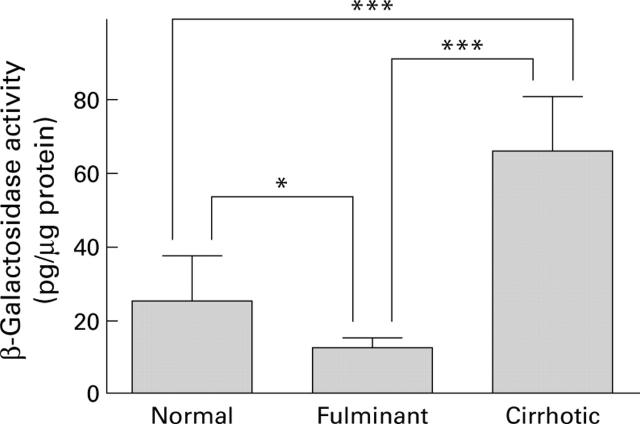
Figure 3 .
Quantitative estimation of lacZ expression in the liver by intravenous administration of adenoviruses. Mice with normal, cirrhotic, and fulminant hepatitis livers were infused intravenously with adenoviruses (2×108 pfu/200 µl) carrying the lacZ gene. Animals were killed on day 4 after adenoviral infusion and lacZ gene expression was quantitatively estimated using the chemiluminescent reporter gene assay system. Each group consisted of eight animals. *0.01<p<0.05, ***p<0.001.
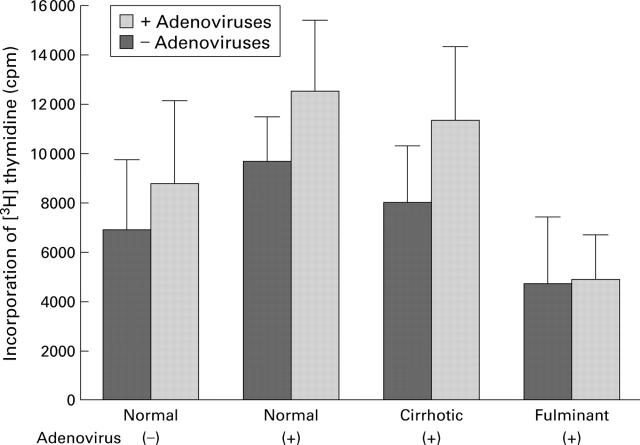
Figure 4 .
Cellular immune responses to adenoviral administration. To examine cellular immune responses to intravenous administration of adenoviruses, splenic cells were collected not only from naive mice that had normal livers and were not given the adenoviral infusion but also from mice with normal, cirrhotic, or fulminant hepatitis livers and given adenoviral infusion. Splenic cells were incubated with or without heat inactivated adenoviruses for five days and pulsed with [3H] thymidine for the last 18 hours of incubation. Each bar represents the mean (SD) of eight animals. There were no significant differences in proliferation of splenic cells between adenovirus stimulated and unstimulated cells in all groups. There were also no significant differences in proliferation among adenovirus stimulated splenic cells collected from naive mice and adenovirus treated mice with normal, cirrhotic, and fulminant hepatitis livers.
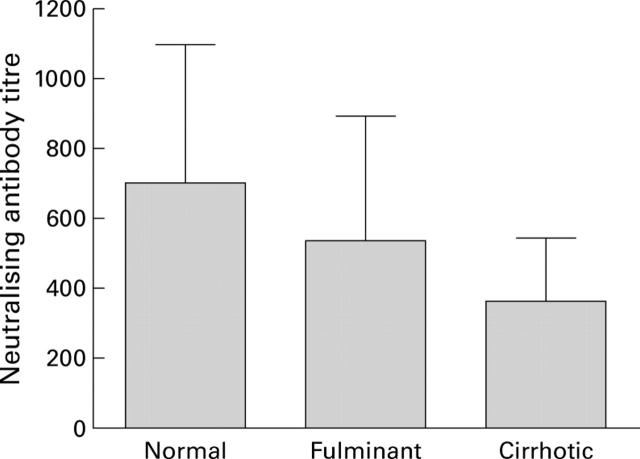
Figure 5 .
Humoral immune responses to adenoviral administration. To examine the development of neutralising antibodies against adenoviruses, serum samples collected from mice with normal, cirrhotic, and fulminant hepatitis livers were decomplemented, serially diluted, and analysed for neutralising antibodies against adenovirus serotype 5. The titre of neutralising antibody for each sample is expressed as the reciprocal dilution of serum that inhibited adenoviral infection by 50%. Each bar represents the mean (SD) of eight animals. There were no significant differences in titres of neutralising antibodies between groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergelson J. M., Krithivas A., Celi L., Droguett G., Horwitz M. S., Wickham T., Crowell R. L., Finberg R. W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998 Jan;72(1):415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotodihardjo A. E., Tait N., Weltman M. D., Liddle C., Little J. M., Farrell G. C. Hepatocellular carcinoma in western Sydney. Aetiology, changes in incidence, and opportunities for better outcomes. Med J Aust. 1994 Oct 3;161(7):433–435. [PubMed] [Google Scholar]
- Cao G., Kuriyama S., Du P., Sakamoto T., Kong X., Masui K., Qi Z. Complete regression of established murine hepatocellular carcinoma by in vivo tumor necrosis factor alpha gene transfer. Gastroenterology. 1997 Feb;112(2):501–510. doi: 10.1053/gast.1997.v112.pm9024304. [DOI] [PubMed] [Google Scholar]
- Cao G., Kuriyama S., Du P., Sakamoto T., Yang W., Masui K., Qi Z. Construction of retroviral vectors to induce strong hepatoma cell-specific expression of cytokine genes. J Gastroenterol Hepatol. 1996 Nov;11(11):1053–1061. doi: 10.1111/j.1440-1746.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Cao G., Kuriyama S., Tsujinoue H., Chen Q., Mitoro A., Qi Z. A novel approach for inducing enhanced and selective transgene expression in hepatocellular-carcinoma cells. Int J Cancer. 2000 Jul 15;87(2):247–252. [PubMed] [Google Scholar]
- Castell J. V., Hernández D., Gómez-Foix A. M., Guillén I., Donato T., Gómez-Lechón M. J. Adenovirus-mediated gene transfer into human hepatocytes: analysis of the biochemical functionality of transduced cells. Gene Ther. 1997 May;4(5):455–464. doi: 10.1038/sj.gt.3300416. [DOI] [PubMed] [Google Scholar]
- Chawla Y., Santa N., Dhiman R. K., Dilawari J. B. Portal hemodynamics by duplex Doppler sonography in different grades of cirrhosis. Dig Dis Sci. 1998 Feb;43(2):354–357. doi: 10.1023/a:1018814624307. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Fierer J. D-Galactosamine hepatotoxicity is associated with endotoxin sensitivity and mediated by lymphoreticular cells in mice. Gastroenterology. 1985 Jan;88(1 Pt 1):115–121. doi: 10.1016/s0016-5085(85)80142-6. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., McElvaney N. G., Rosenfeld M. A., Chu C. S., Mastrangeli A., Hay J. G., Brody S. L., Jaffe H. A., Eissa N. T., Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994 Sep;8(1):42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- Crystal R. G. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995 Oct 20;270(5235):404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- Dai Y., Schwarz E. M., Gu D., Zhang W. W., Sarvetnick N., Verma I. M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J., Lochmüller H., Acsadi G., Jani A., Holland P., Guérin C., Massie B., Karpati G. Differential short-term transduction efficiency of adult versus newborn mouse tissues by adenoviral recombinants. Exp Mol Pathol. 1995 Apr;62(2):131–143. doi: 10.1006/exmp.1995.1015. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993 Aug;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Kanai F., Lan K. H., Shiratori Y., Tanaka T., Ohashi M., Okudaira T., Yoshida Y., Wakimoto H., Hamada H., Nakabayashi H. In vivo gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res. 1997 Feb 1;57(3):461–465. [PubMed] [Google Scholar]
- Kanai F., Shiratori Y., Yoshida Y., Wakimoto H., Hamada H., Kanegae Y., Saito I., Nakabayashi H., Tamaoki T., Tanaka T. Gene therapy for alpha-fetoprotein-producing human hepatoma cells by adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Hepatology. 1996 Jun;23(6):1359–1368. doi: 10.1002/hep.510230611. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Hallenbeck P., Kotani T., Nakabayashi H., McGarrity G., Tamaoki T., Anderson W. F., Chiang Y. L. Adenovirus-mediated gene therapy of hepatocellular carcinoma using cancer-specific gene expression. Cancer Res. 1995 Nov 15;55(22):5283–5287. [PubMed] [Google Scholar]
- Kaplan J. M., Smith A. E. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997 Jun 10;8(9):1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Landen C. N., Rothenberg S. R., Taylor L. A., Leland F., Wiehle S., Fang B., Bellinger D., Finegold M., Thompson A. R. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., McKinley D. R., Austin L. L., Raper S. E., Stratford-Perricaudet L. D., Wilson J. M. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994 May 6;269(18):13695–13702. [PubMed] [Google Scholar]
- Kuriyama S., Kikukawa M., Masui K., Okuda H., Nakatani T., Akahane T., Mitoro A., Tominaga K., Tsujinoue H., Yoshiji H. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999 Oct 29;83(3):374–380. doi: 10.1002/(sici)1097-0215(19991029)83:3<374::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Kikukawa M., Masui K., Okuda H., Nakatani T., Sakamoto T., Yoshiji H., Fukui H., Ikenaka K., Mullen C. A. Cytosine deaminase/5-fluorocytosine gene therapy can induce efficient anti-tumor effects and protective immunity in immunocompetent mice but not in athymic nude mice. Int J Cancer. 1999 May 17;81(4):592–597. doi: 10.1002/(sici)1097-0215(19990517)81:4<592::aid-ijc15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Masui K., Kikukawa M., Sakamoto T., Nakatani T., Nagao S., Yamazaki M., Yoshiji H., Tsujinoue H., Fukui H. Complete cure of established murine hepatocellular carcinoma is achievable by repeated injections of retroviruses carrying the herpes simplex virus thymidine kinase gene. Gene Ther. 1999 Apr;6(4):525–533. doi: 10.1038/sj.gt.3300869. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Mitoro A., Yamazaki M., Tsujinoue H., Nakatani T., Akahane T., Toyokawa Y., Kojima H., Okamoto S., Fukui H. Comparison of gene therapy with the herpes simplex virus thymidine kinase gene and the bacterial cytosine deaminase gene for the treatment of hepatocellular carcinoma. Scand J Gastroenterol. 1999 Oct;34(10):1033–1041. doi: 10.1080/003655299750025156. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Nakatani T., Masui K., Sakamoto T., Tominaga K., Yoshikawa M., Fukui H., Ikenaka K., Tsujii T. Bystander effect caused by suicide gene expression indicates the feasibility of gene therapy for hepatocellular carcinoma. Hepatology. 1995 Dec;22(6):1838–1846. [PubMed] [Google Scholar]
- Kuriyama S., Nakatani T., Masui K., Sakamoto T., Tominaga K., Yoshikawa M., Fukui H., Ikenaka K., Tsujii T. Evaluation of prodrugs ability to induce effective ablation of cells transduced with viral thymidine kinase gene. Anticancer Res. 1996 Sep-Oct;16(5A):2623–2628. [PubMed] [Google Scholar]
- Kuriyama S., Sakamoto T., Masui K., Nakatani T., Tominaga K., Kikukawa M., Yoshikawa M., Ikenaka K., Fukui H., Tsujii T. Tissue-specific expression of HSV-tk gene can induce efficient antitumor effect and protective immunity to wild-type hepatocellular carcinoma. Int J Cancer. 1997 May 2;71(3):470–475. doi: 10.1002/(sici)1097-0215(19970502)71:3<470::aid-ijc27>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Tominaga K., Kikukawa M., Nakatani T., Tsujinoue H., Yamazaki M., Nagao S., Toyokawa Y., Mitoro A., Fukui H. Inhibitory effects of human sera on adenovirus-mediated gene transfer into rat liver. Anticancer Res. 1998 Jul-Aug;18(4A):2345–2351. [PubMed] [Google Scholar]
- Kuriyama S., Tominaga K., Kikukawa M., Tsujimoto T., Nakatani T., Tsujinoue H., Okuda H., Nagao S., Mitoro A., Yoshiji H. Transient cyclophosphamide treatment before intraportal readministration of an adenoviral vector can induce re-expression of the original gene construct in rat liver. Gene Ther. 1999 May;6(5):749–757. doi: 10.1038/sj.gt.3300894. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Tominaga K., Mitoro A., Tsujinoue H., Nakatani T., Yamazaki M., Nagao S., Toyokawa Y., Okamoto S., Fukui H. Immunomodulation with FK506 around the time of intravenous re-administration of an adenoviral vector facilitates gene transfer into primed rat liver. Int J Cancer. 2000 Mar 15;85(6):839–844. doi: 10.1002/(sici)1097-0215(20000315)85:6<839::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Yoshikawa M., Ishizaka S., Tsujii T., Ikenaka K., Kagawa T., Morita N., Mikoshiba K. A potential approach for gene therapy targeting hepatoma using a liver-specific promoter on a retroviral vector. Cell Struct Funct. 1991 Dec;16(6):503–510. doi: 10.1247/csf.16.503. [DOI] [PubMed] [Google Scholar]
- Miyake S., Makimura M., Kanegae Y., Harada S., Sato Y., Takamori K., Tokuda C., Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve D. A., Barnes M. J., Stillman I. E., Libermann T. A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999 Apr 10;10(6):965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Akiyoshi H., Saito I., Sato K. Adenovirus-mediated gene expression in the septal cells of cirrhotic rat livers. J Hepatol. 1999 Jan;30(1):101–106. doi: 10.1016/s0168-8278(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Wakimoto H., Abe J., Kanegae Y., Saito I., Aoyagi M., Hirakawa K., Hamada H. Adoptive immunotherapy with murine tumor-specific T lymphocytes engineered to secrete interleukin 2. Cancer Res. 1994 Nov 15;54(22):5757–5760. [PubMed] [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991 Dec 15;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Sullivan D. E., Dash S., Du H., Hiramatsu N., Aydin F., Kolls J., Blanchard J., Baskin G., Gerber M. A. Liver-directed gene transfer in non-human primates. Hum Gene Ther. 1997 Jul 1;8(10):1195–1206. doi: 10.1089/hum.1997.8.10-1195. [DOI] [PubMed] [Google Scholar]
- Yang Y., Greenough K., Wilson J. M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996 May;3(5):412–420. [PubMed] [Google Scholar]
- Zabner J., Couture L. A., Gregory R. J., Graham S. M., Smith A. E., Welsh M. J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993 Oct 22;75(2):207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]