Abstract
BACKGROUND AND AIMS—The gastrointestinal microflora exerts a barrier effect against enteropathogens. The aim of this study was to examine if bifidobacteria, a major species of the human colonic microflora, participates in the barrier effect by developing antimicrobial activity against enterovirulent bacteria. METHODS—Antibacterial activity was examined in vitro against a wide range of Gram negative and Gram positive pathogens. Inhibition of Salmonella typhimurium SL1334 cell association and cell invasion was investigated in vitro using Caco-2 cells. Colonisation of the gastrointestinal tract in vivo by bifidobacteria was examined in axenic C3/He/Oujco mice. Antimicrobial activity was examined in vivo in axenic C3/He/Oujco mice infected by the lethal S typhimurium C5 strain. RESULTS—Fourteen human bifidobacterium strains isolated from infant stools were examined for antimicrobial activity. Two strains (CA1 and F9) expressed antagonistic activity against pathogens in vitro, inhibited cell entry, and killed intracellular S typhimurium SL1344 in Caco-2 cells. An antibacterial component(s) produced by CA1 and F9 was found to be a lipophilic molecule(s) with a molecular weight of less than 3500. In the axenic C3/He/Oujco mice, CA1 and F9 strains colonised the intestinal tract and protected mice against S typhimurium C5 lethal infection. CONCLUSION—Several bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity, suggesting that they could participate in the "barrier effect" produced by the indigenous microflora. Keywords: bifidobacteria; infant microflora; gastrointestinal infection; antimicrobial; microbial infection; intestinal cells
Full Text
The Full Text of this article is available as a PDF (146.6 KB).
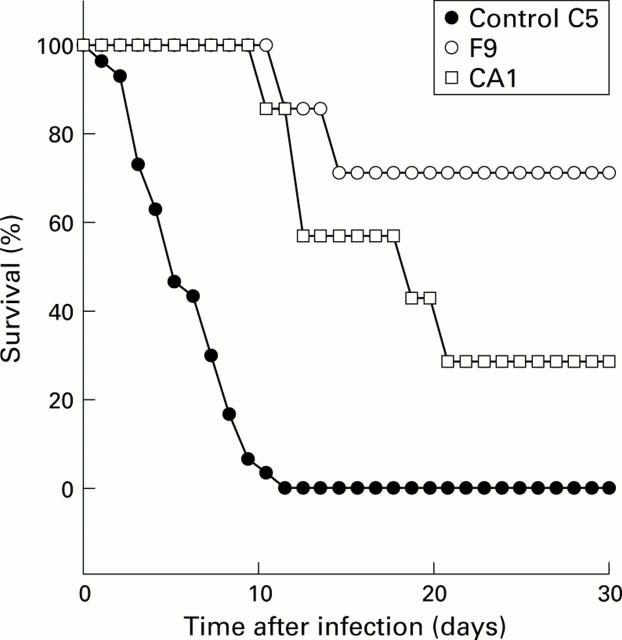
Figure 1 .
Protective effect of human infant bifidobacterium CA1 and F9 strains established in germ free C3H/He/Oujco mice against S typhimurium lethal infection. Experimental conditions are described in materials and methods.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benno Y., Sawada K., Mitsuoka T. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol. 1984;28(9):975–986. doi: 10.1111/j.1348-0421.1984.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Berg R. D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996 Nov;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- Bernet-Camard M. F., Liévin V., Brassart D., Neeser J. R., Servin A. L., Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997 Jul;63(7):2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J. R., Servin A. L. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993 Dec;59(12):4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J. R., Servin A. L. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994 Apr;35(4):483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg L., Henriksson A., Conway P. L. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol. 1993 Jan;59(1):34–39. doi: 10.1128/aem.59.1.34-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee-Oudenhoven I., Termont D., Dekker R., Van der Meer R. Calcium in milk and fermentation by yoghurt bacteria increase the resistance of rats to Salmonella infection. Gut. 1996 Jan;38(1):59–65. doi: 10.1136/gut.38.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C. L., Willis A. T. Resistance of the breast-fed infant to gastroenteritis. Br Med J. 1971 Aug 7;3(5770):338–343. doi: 10.1136/bmj.3.5770.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. C., Reid G., Irvin R. T., Bruce A. W., Costerton J. W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985 Jan;47(1):84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvière G., Coconnier M. H., Kerneis S., Darfeuille-Michaud A., Joly B., Servin A. L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol Lett. 1992 Mar 15;70(3):213–217. doi: 10.1016/0378-1097(92)90700-x. [DOI] [PubMed] [Google Scholar]
- Coconnier M. H., Bernet M. F., Kernéis S., Chauvière G., Fourniat J., Servin A. L. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett. 1993 Jul 1;110(3):299–305. doi: 10.1111/j.1574-6968.1993.tb06339.x. [DOI] [PubMed] [Google Scholar]
- Coconnier M. H., Lievin V., Hemery E., Servin A. L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998 Nov;64(11):4573–4580. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coconnier M. H., Liévin V., Bernet-Camard M. F., Hudault S., Servin A. L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997 May;41(5):1046–1052. doi: 10.1128/aac.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveillard M., Fourel V., Barc M. C., Kernéis S., Coconnier M. H., Karjalainen T., Bourlioux P., Servin A. L. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol Microbiol. 1993 Feb;7(3):371–381. doi: 10.1111/j.1365-2958.1993.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Hashiba H., Hirota T., Forstner J. F. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl Environ Microbiol. 1997 Feb;63(2):506–512. doi: 10.1128/aem.63.2.506-512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R., Gibson G. R. Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol Suppl. 1997;222:28–31. doi: 10.1080/00365521.1997.11720714. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Beatty E. R., Wang X., Cummings J. H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995 Apr;108(4):975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994 Oct;77(4):412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Hudault S., Bridonneau C., Raibaud P., Chabanet C., Vial M. F. Relationship between intestinal colonization of Bifidobacterium bifidum in infants and the presence of exogenous and endogenous growth-promoting factors in their stools. Pediatr Res. 1994 Jun;35(6):696–700. doi: 10.1203/00006450-199406000-00015. [DOI] [PubMed] [Google Scholar]
- Hudault S., Liévin V., Bernet-Camard M. F., Servin A. L. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol. 1997 Feb;63(2):513–518. doi: 10.1128/aem.63.2.513-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Freter R. Control of Escherichia coli populations by a combination of indigenous clostridia and lactobacilli in gnotobiotic mice and continuous-flow cultures. Infect Immun. 1989 Feb;57(2):559–565. doi: 10.1128/iai.57.2.559-565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K., Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- McCartney A. L., Wenzhi W., Tannock G. W. Molecular analysis of the composition of the bifidobacterial and lactobacillus microflora of humans. Appl Environ Microbiol. 1996 Dec;62(12):4608–4613. doi: 10.1128/aem.62.12.4608-4613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen E. N., Bonneville F., Fauchère J. L. Modification par l'érythromycine et un extrait de Lactobacillus acidophilus de la colonisation de l'intestin et de la translocation de Campylobacter jejuni chez la souris axénique. Ann Inst Pasteur Microbiol. 1986 Mar-Apr;137A(2):199–207. doi: 10.1016/s0769-2609(86)80024-2. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Selsted M. E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996 Sep;10(11):1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Ramare F., Nicoli J., Dabard J., Corring T., Ladire M., Gueugneau A. M., Raibaud P. Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl Environ Microbiol. 1993 Sep;59(9):2876–2883. doi: 10.1128/aem.59.9.2876-2883.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M., Bauman N. A., Oung I., Perman J. A., Yolken R. H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994 Oct 15;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- Salminen S., Isolauri E., Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Van Leeuwenhoek. 1996 Oct;70(2-4):347–358. doi: 10.1007/BF00395941. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M., Jacobus N. V., Deneke C., Gorbach S. L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987 Aug;31(8):1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancrède C. Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis. 1992 Nov;11(11):1012–1015. doi: 10.1007/BF01967791. [DOI] [PubMed] [Google Scholar]
- Velraeds M. M., van der Mei H. C., Reid G., Busscher H. J. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol. 1996 Jun;62(6):1958–1963. doi: 10.1128/aem.62.6.1958-1963.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Machii K., Tsuyuki S., Momose H., Kawashima T., Ueda K. Immunological responses to monoassociated Bifidobacterium longum and their relation to prevention of bacterial invasion. Immunology. 1985 Sep;56(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- Yasui H., Kiyoshima J., Ushijima H. Passive protection against rotavirus-induced diarrhea of mouse pups born to and nursed by dams fed Bifidobacterium breve YIT4064. J Infect Dis. 1995 Aug;172(2):403–409. doi: 10.1093/infdis/172.2.403. [DOI] [PubMed] [Google Scholar]